D-dimers are a determinant of hypercoagulable state and have been found to be related to acute coronary syndromes. We aimed to establish the association between increased D-dimer levels and coronary artery disease (CAD) severity using SYNTAX Score (SS) II in patients with ST elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI).
MethodsThis retrospective study included 300 consecutive patients (81.7% males, mean age 55±12 years) with STEMI who underwent a primary PCI. Patients were divided into two groups according to their median SSII [SSII<25 as a low group (n=151) and SSII≥25 as a high group (n=149)]. Blood samples for D-dimers and the other biochemical parameters were obtained from each patient at admission.
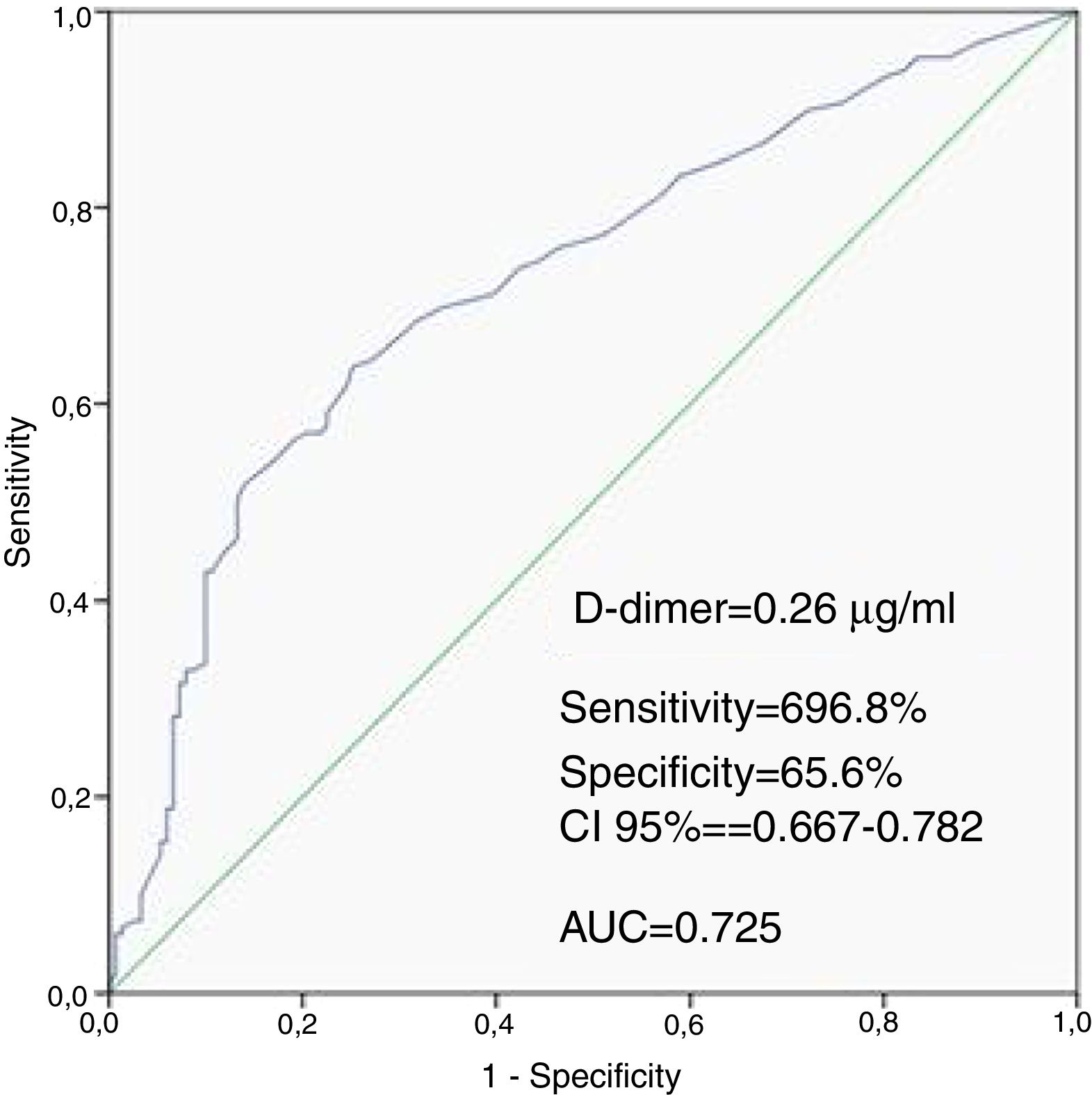
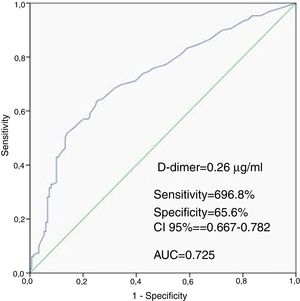
ResultsWhen compared with the low SSII group, frequency of female gender, no-reflow phenomenon, D-dimer levels, thrombus score, creatine kinase MB and troponin were significantly higher, whereas left ventricular ejection fraction (LVEF) and glomerular filtration rate (GFR) were lower in the high SSII group (p<0.05, for all). D-dimer levels, thrombus score, LVEF, GFR and no-reflow phenomenon were independent predictors of CAD severity (p<0.05, for all). Receiver operating characteristic curve analysis showed that the D-dimer cut-off value for predicting the severity of CAD was 0.26 μg/ml (69.8% sensitivity and 65.6% specificity, p<0.001).
ConclusionIncreased D-dimer levels are associated with the severity of CAD based on Syntax Score II, in patients with STEMI who successfully underwent revascularization with a primary PCI.
Os D-dímeros elevados refletem um estado de hipercoagulabilidade determinante que se relaciona com as síndromes coronárias agudas (SCA). Foi nosso objetivo determinar a associação entre os níveis elevados de D-dímeros e a gravidade da doença coronária utilizando o score SYNTAX (SS) em doentes com enfarte do miocárdio com elevação do segmento ST (STEMI) submetidos a intervenção coronária percutânea (ICP).
MétodosEste estudo retrospetivo incluiu 300 doentes consecutivos (homens – 81,7% com média de 55±12 anos) com STEMI submetidos a ICP. Os doentes foram divididos em dois grupos de acordo com o SSII médio [SSII<25 no grupo de baixo risco (n=151) e SSII≥25 no grupo de alto risco (n=149)]. As análises de sangue para avaliação dos D-dímeros e de outros parâmetros bioquímicos foram efetuadas durante a admissão hospitalar em todos os doentes.
ResultadosNo grupo SSII de alto risco, o género feminino, o «fenómeno no-reflow», os níveis dos D-dímeros, o score de trombos, a creatinaquinase MB (CK-MB) e a troponina foram significativamente superiores aos grupos SSII de baixo risco (p<0,05); a fração de ejeção ventricular esquerda (FEVE) e a taxa de filtração glomerular (TFG) foram inferiores (p<0,05) aos do grupo de baixo risco de SSII. Os níveis dos D-dímeros, o score de trombos, a FEVE, a TFG o fenómeno no-reflow foram fatores predizentes independentes da gravidade da doença coronária (p<0,05 em todos). A análise da curva ROC (receiver operating characteristic curve) demonstrou que o valor cut-off dos D-dímeros para prognóstico da gravidade doença coronária foi de 0,26 μg/ml (sensibilidade 69,8% e especificidade 65,6%, p<0,001).
ConclusãoO nível D-dímeros está associado à gravidade da doença coronária avaliada pelo Score Syntax II em doentes com STEMI, bem-sucedidos na revascularização com ICP.
Myocardial infarction (MI) usually originates from thrombosis of atherosclerotic plaque rupture.1 Circulating markers of thrombosis, activated coagulation and fibrinolysis may be linked to the pathogenesis of coronary artery disease (CAD).1,2 D-dimers are a specific product of fibrin degradation that results from thrombin activation, activated factor XIII, and plasmin.3 It has been demonstrated that higher levels of D-dimer in plasma are one of the most useful biomarkers for acute MI due to plaque rupture induced coronary thrombosis, which plays an important role in the pathophysiology of acute MI.4 Moreover, D-dimer levels may reflect a systemic prothrombotic state and focal vessel wall-related fibrin formation with unstable atherosclerotic plaque activity.5 Recent studies have also shown that elevated D-dimer levels are associated with in-hospital and mid-term mortality in patients with ST elevation myocardial infarction (STEMI).6
The SYNTAX score (SS) is an angiographic lesion-based scoring system originally designed to assess the complexity of CAD.7 The SYNTAX score II (SSII) includes clinical variables determined by applying a Cox proportional hazards model to the results of the SYNTAX trial.8 In a study investigating the predictive value of the SSII compared to the SS, SSII had better prognostic accuracy for patients with CAD.9 Furthermore, both SS and SSII were associated with long-term mortality and major adverse cardiac events in patients with STEMI.10,11
In the present study, we investigated the association between D-dimers and the severity of CAD using the SS and SSII in patients with STEMI treated with primary percutaneous coronary intervention (PCI).
MethodsStudy populationWe included 300 consecutive patients (81.7% males, mean age 55±12 years) with STEMI who underwent primary PCI. Patients with a history of coronary artery bypass graft (CABG) surgery, malignancy, severe liver or renal disease, significant hematologic (anemia, thrombocytopenia, leukocytosis, leukemia) and febrile disorders (body temperature more than 38°C), and anticoagulant therapy (heparin, low-molecular-weight heparin, warfarin) before admission were excluded from the study. Patients were divided into two groups according to their median SSII. Patients with SSII<25 were included in the low SSII group (n=151, 94.7% males) and patients with SSII≥25 were included in the high SSII group (n=149, 68.5% males). STEMI was defined based on the following criteria: ongoing ischemic symptoms (within 12 hours), typical rise or fall in cardiac biomarkers and a new ST elevation in two or more contiguous leads with leads V1, V2, and V3 measuring at least 0.2 mV or at least 0.1 mV in the remaining leads, or a newly developed left bundle-branch block pattern.12
Baseline characteristics of patients with STEMI were recorded, such as age, gender, smoking status at admission, history of hypertension, and history of diabetes. Left ventricular ejection fraction (LVEF) was determined using the Simpson method, following guidance from the American Society of Echocardiography.13 The study was conducted according to the recommendations set forth by the Declaration of Helsinki on Biomedical Research Involving Human Subjects. The institutional ethics committee approved the study protocol and each participant provided written informed consent.
Blood samplingVenous blood samples were obtained before primary PCI at admission. White blood count (WBC) and differential counts were measured. Total WBC, neutrophils, monocytes and lymphocytes were measured using a Sysmex K-1000 (Block Scientific, Bohemia, New York, USA) autoanalyzer within five minutes of sampling. Plasma triglyceride, low-density lipoprotein, high-density lipoprotein, glucose, uric acid, and creatinine concentrations were measured with an automated chemistry analyzer (Abbott Aeroset, Minnesota, USA) using commercial kits (Abbott). Creatine kinase MB (CK-MB) activity was measured with an assay that used two monoclonal antibodies (CK-MB STAT) on an Elecsys 2010 analyzer (Roche Diagnostics, Basel, Switzerland) by electrochemiluminescence immunoassay. The glomerular filtration rate (GFR) was estimated using the Cockroft-Gault formula. Blood samples for D-dimer analysis were obtained during the initial assessment of patients in the emergency department. The D-dimer test is a particle enhanced immuno-turbidimetric test. The test is based on fixed time determination of the D-dimer concentration using photometric measurement of the antigen-antibody-reaction between antibodies against D-dimer bound to particles and D-dimer present in the sample. During plasma coagulation soluble fibrin is generated by the influence of thrombin on fibrinogen. Soluble fibrin is cross-linked to vessel walls by factor XIIIa. When splitting this cross-linked fibrin characteristic products called D-dimers are released.
Coronary angiography and calculation of Syntax score and Syntax score IIAll patients underwent selective coronary angiography using the Judkins technique. All patients received, on a routine basis, 300 mg acetylsalicylic acid and a 600 mg loading dose of clopidogrel before the intervention and unfractionated heparin during the intervention. Culprit lesions were treated with stent implantation and balloon angioplasty, if necessary. Coronary blood flow patterns before and after primary PCI were thoroughly assessed on the basis of thrombolysis in myocardial infarction (TIMI) flow grade using grades 0, 1, 2, and 3.14 Thrombus burden on angiography was classified as follows: Grade 0: no thrombus; Grade 1: possible thrombus; Grade 2: the largest dimension of the thrombus is <1/2 vessel diameter; Grade 3: largest dimension >1/2 to <2 vessel diameters; Grade 4: greatest dimension >2 vessel diameters; Grade 5: total vessel occlusion due to thrombus.15 Patients were stratified into low thrombus burden (Grades 1, 2 and 3) and high thrombus burden groups (4 and 5) according to final thrombus score. We defined angiographic no-reflow as a coronary TIMI flow grade 2 after a vessel had been re-canalized or TIMI flow grade 3 together with a final myocardial blush grade (MBG) 2 as previously described.16
Each lesion ≥1.5 mm in diameter and with ≥50% stenosis was scored using the online SS Calculator, version 2.1 (www.syntaxscore.com). Since patients with STEMI were excluded from the initial SS algorithm, we defined an occluded infarct-related artery as an occluded artery of <3 months duration, as reported in previous studies of STEMI patients.10 The SSII was also calculated using the on-line calculator (www.syntaxscore.com) and included two anatomical variables (anatomical SS and unprotected left main coronary artery disease) and six clinical variables (age, creatinine clearance, LVEF, sex, chronic obstructive pulmonary disease (COPD), and peripheral arterial disease).8 Two experienced interventional cardiologists, who were blinded to all cases, analyzed the angiograms.
Statistical analysisThe analyses were performed using the SPSS software (Statistical Package for the Social Sciences, Version 22.0, SSPS Inc., Chicago, IL, USA). Descriptive statistics were given as mean ± SD or median (min-max) for continuous variables depending on distribution pattern; categorical variables were summarized as frequency and percentage. The Shapiro-Wilk test was used to examine distribution of continuous data. The independent samples t-test or Mann-Whitney U test was used for group comparisons. Pearson or Spearman correlation analyzes were used to analyze the correlation between continuous variables. Categorical variables were analyzed by Pearson Chi-square or Fisher's Exact test. Multivariate logistic regression analysis (Forward LR) was performed to determine the risk factors for SSII, by taking all variables differ significantly in univariate analyses. A receiver operating characteristic (ROC) curve analysis was carried out to identify the optimal D-dimer cut-off point for predicting CAD severity. The area under the ROC curve was calculated as a measure of the accuracy of the test. A two-tailed p value of <0.05 was considered as significant.
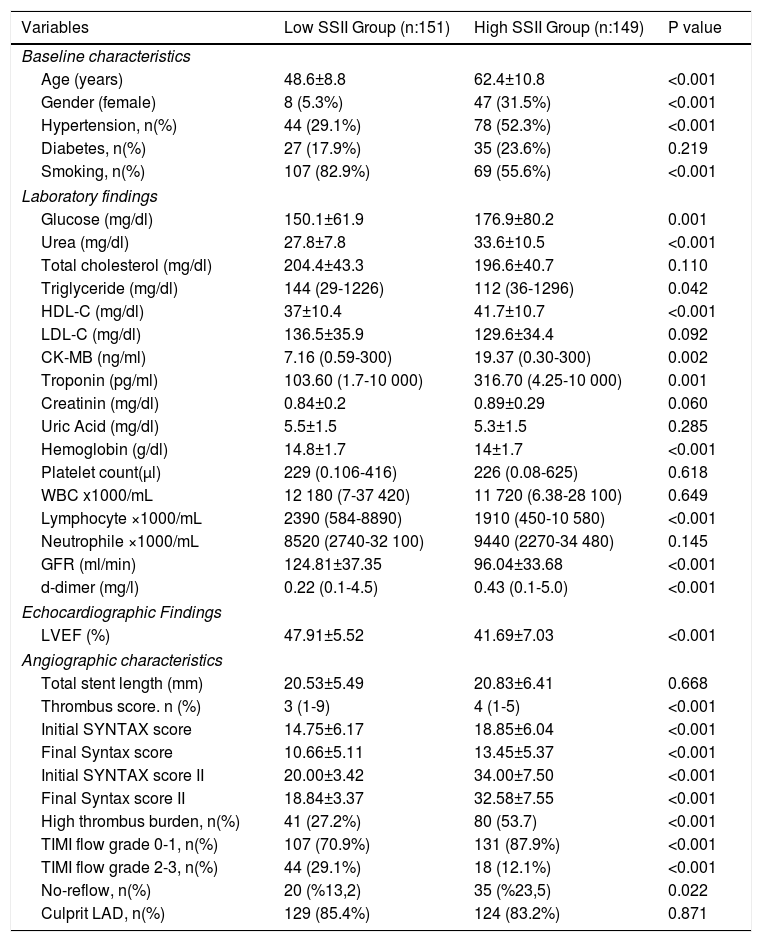
ResultsThe baseline clinical, laboratory, echocardiographic and angiographic characteristics of the study population are listed in Table 1. According to our findings, initial Syntax score, final Syntax score, thrombus score, initial TIMI flow grade 0-1 and no-reflow phenomenon parameters were significantly higher in the high SSII group than in the low SSII group (p<0.05). LVEF was also lower in the high SSII group than in the low SSII group (p<0.001).
Comparison of baseline, laboratory, echocardiographic and angiographic characteristics of groups.
| Variables | Low SSII Group (n:151) | High SSII Group (n:149) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 48.6±8.8 | 62.4±10.8 | <0.001 |
| Gender (female) | 8 (5.3%) | 47 (31.5%) | <0.001 |
| Hypertension, n(%) | 44 (29.1%) | 78 (52.3%) | <0.001 |
| Diabetes, n(%) | 27 (17.9%) | 35 (23.6%) | 0.219 |
| Smoking, n(%) | 107 (82.9%) | 69 (55.6%) | <0.001 |
| Laboratory findings | |||
| Glucose (mg/dl) | 150.1±61.9 | 176.9±80.2 | 0.001 |
| Urea (mg/dl) | 27.8±7.8 | 33.6±10.5 | <0.001 |
| Total cholesterol (mg/dl) | 204.4±43.3 | 196.6±40.7 | 0.110 |
| Triglyceride (mg/dl) | 144 (29-1226) | 112 (36-1296) | 0.042 |
| HDL-C (mg/dl) | 37±10.4 | 41.7±10.7 | <0.001 |
| LDL-C (mg/dl) | 136.5±35.9 | 129.6±34.4 | 0.092 |
| CK-MB (ng/ml) | 7.16 (0.59-300) | 19.37 (0.30-300) | 0.002 |
| Troponin (pg/ml) | 103.60 (1.7-10 000) | 316.70 (4.25-10 000) | 0.001 |
| Creatinin (mg/dl) | 0.84±0.2 | 0.89±0.29 | 0.060 |
| Uric Acid (mg/dl) | 5.5±1.5 | 5.3±1.5 | 0.285 |
| Hemoglobin (g/dl) | 14.8±1.7 | 14±1.7 | <0.001 |
| Platelet count(μl) | 229 (0.106-416) | 226 (0.08-625) | 0.618 |
| WBC x1000/mL | 12 180 (7-37 420) | 11 720 (6.38-28 100) | 0.649 |
| Lymphocyte ×1000/mL | 2390 (584-8890) | 1910 (450-10 580) | <0.001 |
| Neutrophile ×1000/mL | 8520 (2740-32 100) | 9440 (2270-34 480) | 0.145 |
| GFR (ml/min) | 124.81±37.35 | 96.04±33.68 | <0.001 |
| d-dimer (mg/l) | 0.22 (0.1-4.5) | 0.43 (0.1-5.0) | <0.001 |
| Echocardiographic Findings | |||
| LVEF (%) | 47.91±5.52 | 41.69±7.03 | <0.001 |
| Angiographic characteristics | |||
| Total stent length (mm) | 20.53±5.49 | 20.83±6.41 | 0.668 |
| Thrombus score. n (%) | 3 (1-9) | 4 (1-5) | <0.001 |
| Initial SYNTAX score | 14.75±6.17 | 18.85±6.04 | <0.001 |
| Final Syntax score | 10.66±5.11 | 13.45±5.37 | <0.001 |
| Initial SYNTAX score II | 20.00±3.42 | 34.00±7.50 | <0.001 |
| Final Syntax score II | 18.84±3.37 | 32.58±7.55 | <0.001 |
| High thrombus burden, n(%) | 41 (27.2%) | 80 (53.7) | <0.001 |
| TIMI flow grade 0-1, n(%) | 107 (70.9%) | 131 (87.9%) | <0.001 |
| TIMI flow grade 2-3, n(%) | 44 (29.1%) | 18 (12.1%) | <0.001 |
| No-reflow, n(%) | 20 (%13,2) | 35 (%23,5) | 0.022 |
| Culprit LAD, n(%) | 129 (85.4%) | 124 (83.2%) | 0.871 |
CK-MB: creatine kinase myocardial band; HDL-C: high density lipoprotein cholesterol; GFR: glomerular filtration rate; LAD: left anterior descending; LVEF: left ventricular ejection fraction; LDL-C: low density lipoprotein cholesterol; SYNTAX: SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery; WBC: white blood cell.
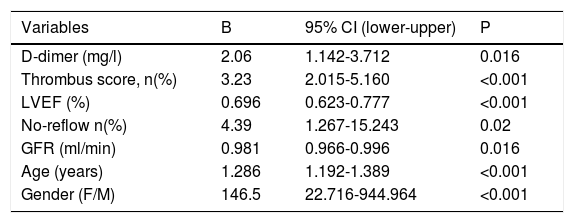
Predictors of CAD severity based on SSII are shown in Table 2. The following were found to be the independent predictors of SSII: D-dimer (β=2.06, CI 95%: 1.142–3.712, p =0.016), thrombus score (β=3.23, CI 95%: 2.015–5.160, p<0.001), LVEF (β=0.696, CI 95%: 0.623–0.777, p<0.001), no-reflow phenomenon (β=4.39, CI 95%: 1.267-15.243, p=0.02), GFR (β=0.981, CI 95%: 0.966-0.996, p=0.02), age (β=1.286, CI 95%: 1.192-1.389, p<0.001), and gender (β=146.5, CI 95%: 22.716-944.964, p<0.001).
Independent predictors of coronary artery disease severity.
| Variables | B | 95% CI (lower-upper) | P |
|---|---|---|---|
| D-dimer (mg/l) | 2.06 | 1.142-3.712 | 0.016 |
| Thrombus score, n(%) | 3.23 | 2.015-5.160 | <0.001 |
| LVEF (%) | 0.696 | 0.623-0.777 | <0.001 |
| No-reflow n(%) | 4.39 | 1.267-15.243 | 0.02 |
| GFR (ml/min) | 0.981 | 0.966-0.996 | 0.016 |
| Age (years) | 1.286 | 1.192-1.389 | <0.001 |
| Gender (F/M) | 146.5 | 22.716-944.964 | <0.001 |
CI: confidence interval; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction.
Patients in the high SSII group had higher levels of D-dimers than the low SSII group. The ROC curve analysis provided a cutoff value of 0.26 μg/ml for D-dimers to predict the severity of CAD with 69.8% sensitivity and 65.6% specificity, with the area under the ROC curve being 0.725 (CI 95%: 0.667-0.782, P<0.001) (Figure 1).
DiscussionTo the best of our knowledge, our study is the first in the literature to assess the association between admission D-dimer levels and the severity of CAD by using SSII in patients with STEMI who underwent primary PCI. We demonstrated that high D-dimer levels were an independent predictor of high SSII in STEMI patients treated with primary PCI. The primary findings of the present study are that D-dimer level, thrombus score, no-reflow phenomenon, LVEF, GFR, age and gender are independent predictors of CAD severity.
Rupture of a vulnerable plaque, which subsequently induces platelet aggregation, triggers acute coronary syndrome (ACS) and coagulation at this site, blocks coronary blood flow and causes myocardial ischemic injury. D-dimer in ACS serves as a direct marker of ongoing fibrinolysis at the site of coronary artery occlusion. Several studies have shown that D-dimers might be helpful in the diagnosis and prognosis of patients with acute chest pain, unstable angina pectoris, and non-ST elevation myocardial infarction (NSTEMI).17,18 Moreover, D-dimer levels rise earlier than cardiac injury markers, including myoglobin in acute ischemic events, because D-dimers are involved at an earlier stage in the pathophysiology of ACS.19 However, data concerning the role of D-dimer testing in STEMI are still unclear. In a previous study, it was reported that when compared with primary PCI, thrombolytic treatment for STEMI was associated with higher levels of D-dimer.20 Besides, Choi et al. observed that the myocardial infarct size of the left ventricle was significantly larger in patients with high D-dimer levels.21 According to these studies, STEMI patients treated with thrombolysis have higher D-dimer levels and a larger myocardial infarct area. Similar to the abovementioned results, we observed that higher CK-MB and troponin levels were correlated with larger infarct size. We also demonstrated that D-dimer levels were significantly higher in the high SSII group.
A growing thrombus partially or completely blocks coronary blood flow during STEMI. High thrombus burden at the site of the infarct-related artery is predictive of distal embolization, which is accepted as the leading cause of no-reflow.22 Since there is a high thrombus burden caused by the coagulation and aggregation process in myocardial infarction, D-dimers, a product of coagulation, are higher in coronary no-reflow or slow-flow phenomenon. Besides, thrombus burden and distal embolization can complicate the success of the operation and patients with a high thrombus burden have poor prognosis. Sarli et al. also reported that D-dimer levels measured at admission might be a predictor of postinterventional no-reflow and in-hospital major adverse cardiovascular events (MACE) in patients with STEMI undergoing primary PCI.23 Accordingly, in the present study, no-reflow phenomenon, which was associated with high D-dimer levels and high thrombus burden, was observed to be an independent predictor of CAD severity.
Akgul et al. showed that six-month cardiovascular mortality was significantly higher in patients with STEMI and high D-dimer levels at admission.6 Additionally, fatal reinfarction, advanced heart failure and MACE were more frequently observed in the high D-dimer group at the mid-term follow-up. Several studies reported that in patients with NSTEMI, D-dimer levels are positively correlated with TIMI and GRACE risk scores, which predict the risk of mortality in NSTEMI.24,25 In the present study, we observed that admission D-dimer levels are an independent predictor of CAD severity based on SSII. Since the severity of CAD correlates with increased cardiovascular mortality, we can speculate that patients with STEMI and higher D-dimer levels on admission are at high risk for cardiovascular mortality and morbidity.
Besides the abovementioned advantages of D-dimer assays, there are some limitations. D-dimer levels are elevated in most patients with acute thrombosis. However, D-dimer levels are also increased following recent surgery, with trauma, advanced age, during pregnancy, puerperium, in malignancy and chronic inflammatory conditions.26 Therefore, D-dimers are a sensitive marker for detection of thrombosis in spite of low specificity. In the present study, D-dimer levels may be found to be elevated as a result of some clinical variables included in SSII, such as age. We performed logistic regression analysis in order to avoid this confusion, and we observed that high D-dimer levels are independently correlated with high SSII.
SYNTAX score, the most commonly used anatomical scoring system worldwide, has been found to be associated with STEMI patient prognosis. SSII is a new scoring system, which includes clinical variables. Karabag et al. reported that SSII was better than SS in the prediction of in-hospital and long-term mortality.27 Therefore, we preferred to use this scoring system in our study.
Recent studies revealed that initial SS is associated with no-reflow phenomenon and long-term mortality in STEMI. Higher SS indicates high thrombus burden, no-reflow risk and higher mortality. Duman et al. reported that SS was higher in patients with STEMI undergoing primary PCI and high thrombus burden.28 Moreover, Yesin et al. showed that SSII might be more useful than SS in prediction of the no-reflow phenomenon.29 Similarly, we observed that thrombus score and no-reflow phenomenon were independent predictors of severe CAD.
ACS occurs three to four times more often in men than in women under the age of 60, however, over the age of 75 women represent the majority of patients.30 In our study, ACS occurs 4.5 times more in men than in women. Female gender was recently reported to be an independent predictor of long-term mortality in the PCI group of the SYNTAX trial, despite adjustment for risk factors.31 Six of the eight SSII variables — anatomical SS, age, LVEF, LMCA disease, COPD, and female gender — showed a moderate to strong interaction effect in predicting long-term mortality in patients with CABG and PCI.8 The authors also demonstrated that female gender has a higher SSII than male gender. Our results were similar to the abovementioned studies in terms of gender and cardiovascular risk.
LimitationsSeveral limitations of the present study should be mentioned. First, our study is limited by its retrospective and observational design, which may diminish its potency. Second, it was a single-center study and the number of patients was limited. Our study focused on the relationship between D-dimer levels and CAD severity as assessed solely by visual assessment of coronary angiograms. Finally, patients with subclinical deep vein thrombosis may have been excluded from the study as we did not routinely perform duplex ultrasound.
ConclusionsIncreased D-dimer levels are associated with CAD severity based on Syntax Score II in patients with STEMI who successfully underwent revascularization with a primary PCI. Moreover, coronary thrombus score, LVEF, no-reflow phenomenon and GFR were other independent predictors of CAD severity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Peer-reviewExternally peer-reviewed.