Coronary artery disease (CAD) is a globally significant cardiovascular condition, ranking among the leading causes of morbidity and mortality. CAD has been predominantly associated with advanced age and classic cardiovascular risk factors. However, over the past decades, there has been a concerning rise in its occurrence among young adults, including patients under 35 years old. The present study analyzes the clinical features and outcomes of patients aged ≤35 years with CAD, compared to two age-matched control groups.
MethodA nested case–control study of ≤35-year-old patients referred for coronary angiography due to clinical suspicion of CAD. Patients were divided into three groups: patients ≤35 years with CAD, subjects ≤35 years without CAD, and young patients ≥36–40 years with CAD.
ResultsOf the 19321 coronary angiographies performed at our center over 10 years, 408 (2.1%) patients were ≤40 years old, 109 patients aged ≤35 years. Risk factors that showed a relationship with the presence of CAD were smoking (OR 2.49; 95% CI 1.03–6.03; p=0.042) and family history of coronary disease (OR 6.70; 95% CI 1.46–30.65; p=0.014). The group aged ≤35 years with CAD exhibited a risk of major cardiovascular adverse events (MACE) (HR 13.3; 95% CI 1.75–100; p<0.001) than subjects ≤35 years without CAD. The probability of major adverse cardiovascular events was associated with being ≤35 years old, diabetes, dyslipidemia, and depression.
ConclusionPatients aged ≤35 exhibited a poor long-term prognosis, with a high risk of new revascularization and acute myocardial infarction during the follow-up period. Focusing on preventive measures can have a significant impact on overall prognosis.
A doença arterial coronária (DAC) é uma condição cardiovascular globalmente significativa, classificada entre as principais causas de morbidade e mortalidade. A DAC tem sido predominantemente associada à idade avançada e aos fatores de risco cardiovasculares clássicos. No entanto, nas últimas décadas, tem havido um aumento preocupante na sua ocorrência entre adultos jovens, incluindo pacientes com menos de 35 anos. O presente estudo analisa as características clínicas e os desfechos de pacientes com idade ≤35 anos com DAC, em comparação com dois grupos controle pareados por idade.
MétodoEstudo caso-controle aninhado de pacientes com idade ≤35 anos encaminhados para cineangiocoronariografia por suspeita clínica de DAC. Os pacientes foram divididos em três grupos: pacientes ≤35 anos com DAC, indivíduos ≤35 anos sem DAC e pacientes jovens ≥36-40 anos com DAC.
ResultadosDas 19.321 coronariografias realizadas no nosso centro num período de 10 anos, 408 (2,1%) pacientes tinham idade ≤40 anos, sendo 109 pacientes com idade ≤35 anos. Os fatores de risco que apresentaram relação com a presença de DAC foram tabagismo (OR 2,49; IC95% 1,03-6,03; p = 0,042) e história familiar de doença coronariana (OR 6,70; IC95% 1,46-30,65; p = 0,014). O grupo com idade ≤35 anos com DAC apresentou risco de MACE (HR 13,3, IC95% 1,75-100; p < 0,001) do que indivíduos ≤35 anos sem DAC. A probabilidade de MACE foi associada a idade ≤35 anos, diabetes, dislipidemia e depressão.
ConclusãoPacientes com idade ≤35 anos apresentaram mau prognóstico a longo prazo, com alto risco de nova revascularização e enfarte agudo do miocárdio durante o período de seguimento. O foco em medidas preventivas pode impactar significativamente o prognóstico geral.
Coronary artery disease (CAD) is a globally significant cardiovascular condition, ranking among the leading causes of morbidity and mortality in the adult population.1,2 CAD has been predominantly associated with advanced age and classic cardiovascular risk factors, such as hypertension, dyslipidemia, smoking, obesity and diabetes.3–5 However, over the past decades, there has been a concerning rise in its occurrence among young adults, including patients under 35 years old.5–7
This worrisome trend of CAD in younger patients has raised the alarm among the medical and scientific community, as its impact may have significant implications for affected individuals and healthcare systems. CAD in younger populations poses unique challenges due to lack of consensus on its pathophysiology, risk factors, and long-term prognosis.9 Therefore, this has a direct impact on patients’ healthcare; they become premature chronic CAD patients, which results in increasing economic and healthcare needs and burden for the community.8 Hence, focusing efforts on a proper understanding and management of CAD should be a priority.
ObjectivesThe aim of this study is to compare three groups of patients undergoing coronary angiography: very young patients (≤35 years) with CAD, subjects aged ≤35 years without CAD, and young patients (≥36–40 years) with CAD. Additionally, we investigated these differences in terms of the number of years lived without major adverse cardiovascular events (MACE) and explored the risk-adjusted association of various risk factors.
MethodsDesign and study populationIt was a single-center, retrospective, nested case–control study in a cohort of ≤40-year-old patients, referred for the first time for coronary angiography due to clinical suspicion (electrocardiographic changes, biomarkers of myocardial injury, or positive ischemia stress test) of CAD, including acute coronary syndrome (ACS) or stable angina. The sample was divided in three groups: (1) patients ≤35 years with CAD (cases), (2) subjects ≤35 years without CAD (control A), and (3) young patients ≥36–40 years with CAD (control B).
Definition of variablesThe variables have been defined according to the diagnosis in the patient's clinical history, categorizing the variables whether or not they have significant CAD, based on coronary angiography, or whether or not they have cardiovascular risk factors, a previous publication provides more information.9 CAD was defined by stenosis ≥75% on angiography or a positive invasive ischemia test or diagnostic of myocardial infarction with nonobstructive coronary arteries (MINORCA).
Ethical and legal aspectsThe investigators participating in this study followed the applicable ethical and legal standards. This study was approved by the Regional Research Ethics Committee with registration code 2015/506.
Statistical analysisDescriptive statistics are reported as mean±standard deviation (SD) or median with interquartile range (IQR) for continuous variables and numbers and percentages for categorical variables. A univariate analysis was performed to detect significant differences between the three groups for the primary variables using the Fisher exact test, χ2 test, Student t test, or Mann–Whitney U test, as appropriate. To determine the risk combination contribution, a multivariate model was used using binary logistic regression analysis. The cardiovascular risk factors analyzed are given as odds ratios (OR) together with their 95% confidence intervals. Kaplan–Meier curves and the log rank test were used to compare the time to the occurrence of a combination of events between the three groups of patients. To identify the factors associated with the combination of events tested, a multivariate Cox regression analysis was performed, calculating the hazard ratio (HR) and 95% confidence intervals. The SPSS program for Windows, version 19, was used for data analysis.
ResultsFrom 1 January 2006 to 31 December 2015, a total of 19321 coronary angiographies were conducted at our center. After applying the exclusion criteria, 299 patients with coronary disease were included in the study, comprising 107 patients aged ≤35 years and 190 patients aged 36–40 years. The control group A consisted of 54 patients aged ≤35 years without CAD in the coronary angiography. At admission, chest pain emerged as the predominant symptom, accounting for ≥94% of the cases in both groups with CAD (cases and control B group). Conversely, the percentage was lower in patients aged ≤35 years without CAD (control A group), at 83.3% (p=0.008). Dyspnea was reported in 11.2% of the ≤35 years group with CAD, 4.7% in the 36–40 years group with CAD, and 13% in the control A group (p=0.048). Regarding the indication for coronary angiography, suspicious acute coronary syndrome was the primary reason in 94 (87.9%) of the patients in the ≤35 years group, 177 (93.2%) in the control B group, and 34 (63%) in the control A group without CAD (p=0.001). The prevalence of MINOCA was 13 (12.1%) and 20 (10.6%) in patients ≤35 years with CAD and 36–40 years group respectively.
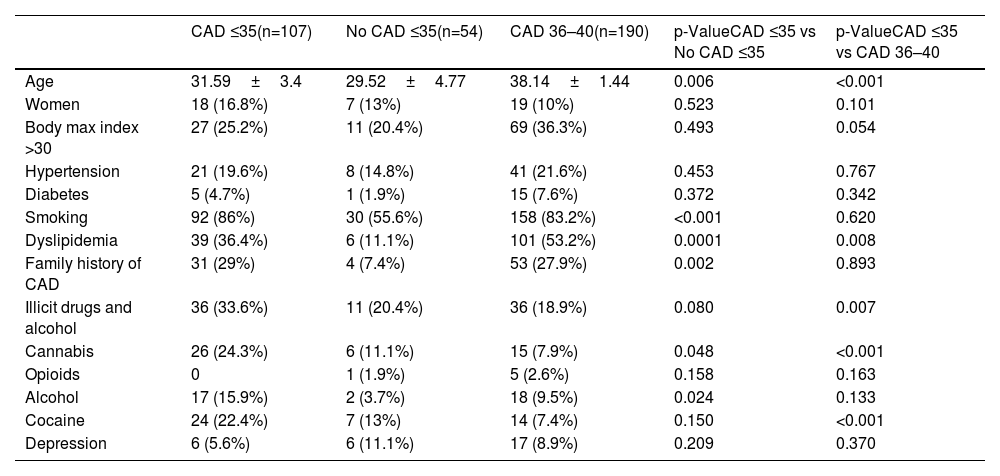
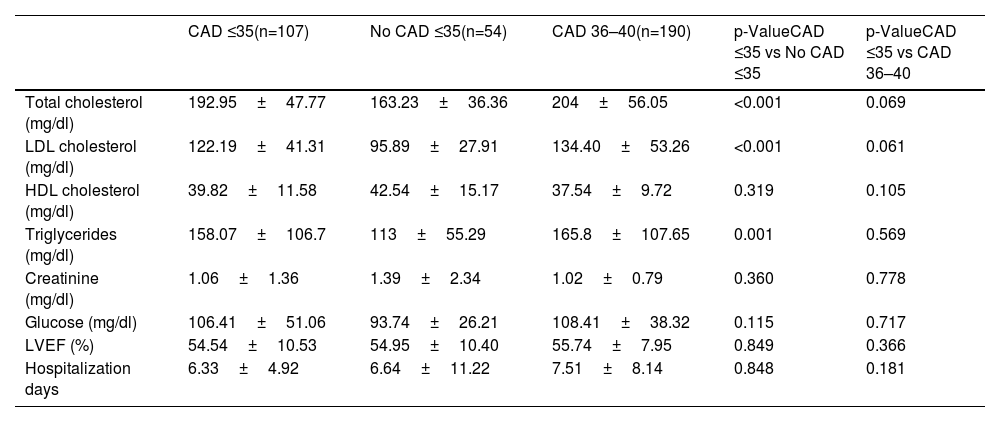
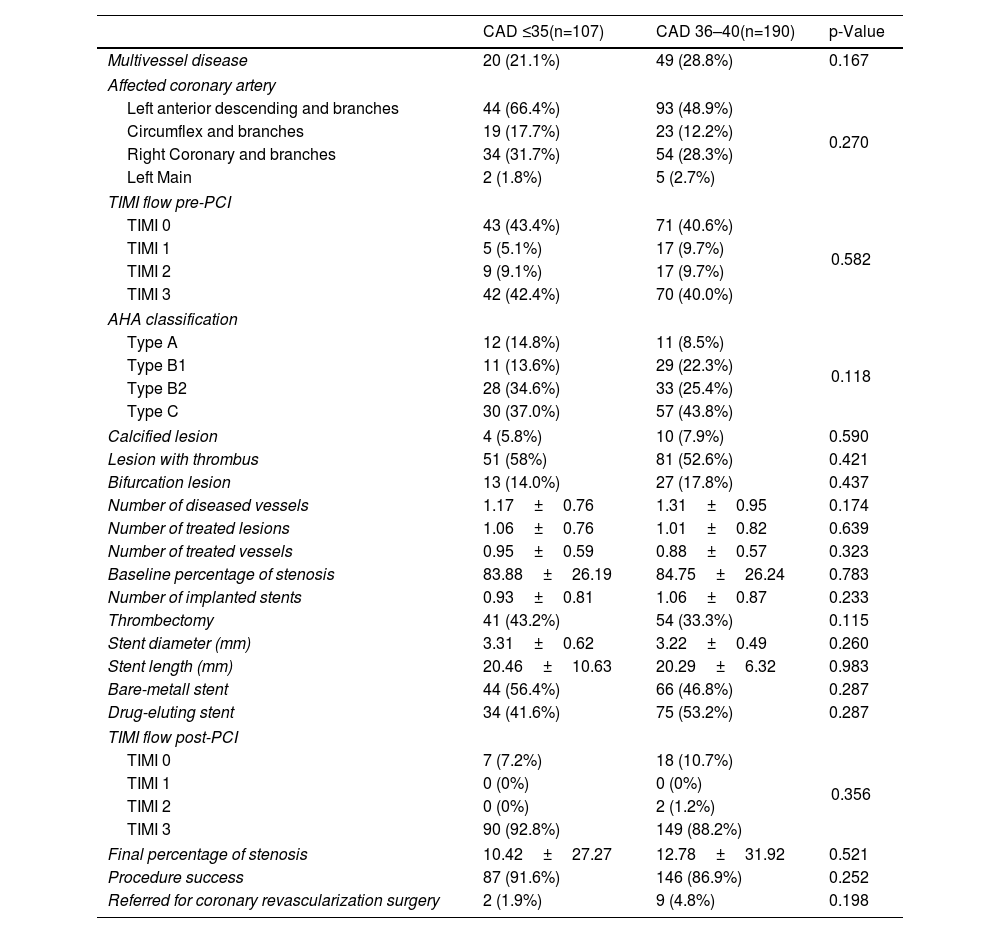
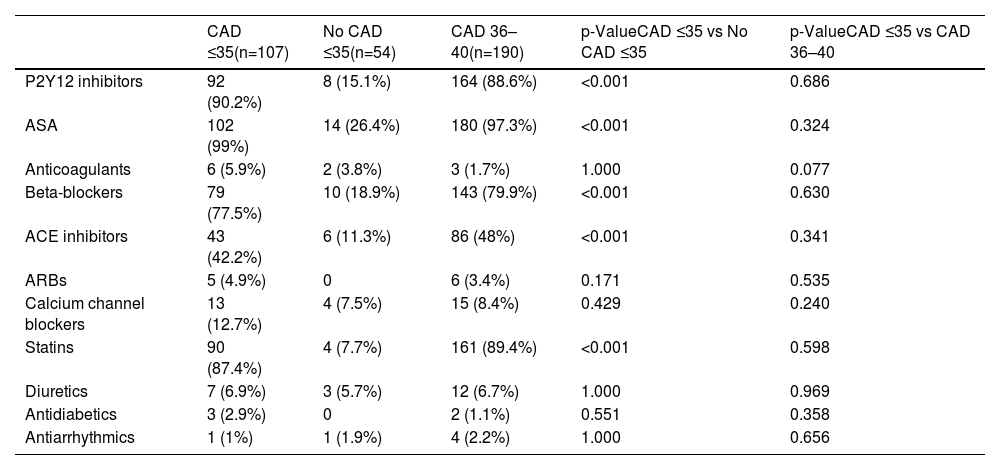
In all study groups, there is a notable predominance of males. Among the identified risk factors, a higher percentage of smokers, individuals with a BMI >30, a positive family history of CAD, and dyslipidemia were prominent in the CAD groups. Statistically significant differences were observed between the groups, not only for dyslipidemia but also for the consumption of toxic substances, as shown in Table 1, other variables presented comparable values across the study groups. No differences were observed between the two groups with CAD with regards laboratory parameters (Table 2). However, significant differences were found between young individuals without CAD and patients aged 35 with ischemic disease. Angiographic characteristics of groups with CAD are displayed in Table 3. Single-vessel CAD was the predominant feature, while 20% of individuals manifest multivessel disease. The left anterior descending coronary artery stands out as the most affected, with a mean stenosis exceeding 80%, showing minimal calcification. Additionally, more than half of the patients presented with thrombus. The pharmacological treatment at hospital discharge is shown in Table 4.
Clinical characteristics of patients ≤35 years old undergoing coronary angiography.
| CAD ≤35(n=107) | No CAD ≤35(n=54) | CAD 36–40(n=190) | p-ValueCAD ≤35 vs No CAD ≤35 | p-ValueCAD ≤35 vs CAD 36–40 | |
|---|---|---|---|---|---|
| Age | 31.59±3.4 | 29.52±4.77 | 38.14±1.44 | 0.006 | <0.001 |
| Women | 18 (16.8%) | 7 (13%) | 19 (10%) | 0.523 | 0.101 |
| Body max index >30 | 27 (25.2%) | 11 (20.4%) | 69 (36.3%) | 0.493 | 0.054 |
| Hypertension | 21 (19.6%) | 8 (14.8%) | 41 (21.6%) | 0.453 | 0.767 |
| Diabetes | 5 (4.7%) | 1 (1.9%) | 15 (7.6%) | 0.372 | 0.342 |
| Smoking | 92 (86%) | 30 (55.6%) | 158 (83.2%) | <0.001 | 0.620 |
| Dyslipidemia | 39 (36.4%) | 6 (11.1%) | 101 (53.2%) | 0.0001 | 0.008 |
| Family history of CAD | 31 (29%) | 4 (7.4%) | 53 (27.9%) | 0.002 | 0.893 |
| Illicit drugs and alcohol | 36 (33.6%) | 11 (20.4%) | 36 (18.9%) | 0.080 | 0.007 |
| Cannabis | 26 (24.3%) | 6 (11.1%) | 15 (7.9%) | 0.048 | <0.001 |
| Opioids | 0 | 1 (1.9%) | 5 (2.6%) | 0.158 | 0.163 |
| Alcohol | 17 (15.9%) | 2 (3.7%) | 18 (9.5%) | 0.024 | 0.133 |
| Cocaine | 24 (22.4%) | 7 (13%) | 14 (7.4%) | 0.150 | <0.001 |
| Depression | 6 (5.6%) | 6 (11.1%) | 17 (8.9%) | 0.209 | 0.370 |
Data are given as number (percentage) or mean±SD.
CAD: coronary artery disease.
Laboratory parameters and quantitative variables.
| CAD ≤35(n=107) | No CAD ≤35(n=54) | CAD 36–40(n=190) | p-ValueCAD ≤35 vs No CAD ≤35 | p-ValueCAD ≤35 vs CAD 36–40 | |
|---|---|---|---|---|---|
| Total cholesterol (mg/dl) | 192.95±47.77 | 163.23±36.36 | 204±56.05 | <0.001 | 0.069 |
| LDL cholesterol (mg/dl) | 122.19±41.31 | 95.89±27.91 | 134.40±53.26 | <0.001 | 0.061 |
| HDL cholesterol (mg/dl) | 39.82±11.58 | 42.54±15.17 | 37.54±9.72 | 0.319 | 0.105 |
| Triglycerides (mg/dl) | 158.07±106.7 | 113±55.29 | 165.8±107.65 | 0.001 | 0.569 |
| Creatinine (mg/dl) | 1.06±1.36 | 1.39±2.34 | 1.02±0.79 | 0.360 | 0.778 |
| Glucose (mg/dl) | 106.41±51.06 | 93.74±26.21 | 108.41±38.32 | 0.115 | 0.717 |
| LVEF (%) | 54.54±10.53 | 54.95±10.40 | 55.74±7.95 | 0.849 | 0.366 |
| Hospitalization days | 6.33±4.92 | 6.64±11.22 | 7.51±8.14 | 0.848 | 0.181 |
Data are given as mean±SD. CAD: coronary artery disease; HDL: high-density lipoproteins; LDL: low-density lipoproteins; LVEF: left ventricle ejection fraction.
Angiographic characteristics of patients with CAD.
| CAD ≤35(n=107) | CAD 36–40(n=190) | p-Value | |
|---|---|---|---|
| Multivessel disease | 20 (21.1%) | 49 (28.8%) | 0.167 |
| Affected coronary artery | |||
| Left anterior descending and branches | 44 (66.4%) | 93 (48.9%) | 0.270 |
| Circumflex and branches | 19 (17.7%) | 23 (12.2%) | |
| Right Coronary and branches | 34 (31.7%) | 54 (28.3%) | |
| Left Main | 2 (1.8%) | 5 (2.7%) | |
| TIMI flow pre-PCI | |||
| TIMI 0 | 43 (43.4%) | 71 (40.6%) | 0.582 |
| TIMI 1 | 5 (5.1%) | 17 (9.7%) | |
| TIMI 2 | 9 (9.1%) | 17 (9.7%) | |
| TIMI 3 | 42 (42.4%) | 70 (40.0%) | |
| AHA classification | |||
| Type A | 12 (14.8%) | 11 (8.5%) | 0.118 |
| Type B1 | 11 (13.6%) | 29 (22.3%) | |
| Type B2 | 28 (34.6%) | 33 (25.4%) | |
| Type C | 30 (37.0%) | 57 (43.8%) | |
| Calcified lesion | 4 (5.8%) | 10 (7.9%) | 0.590 |
| Lesion with thrombus | 51 (58%) | 81 (52.6%) | 0.421 |
| Bifurcation lesion | 13 (14.0%) | 27 (17.8%) | 0.437 |
| Number of diseased vessels | 1.17±0.76 | 1.31±0.95 | 0.174 |
| Number of treated lesions | 1.06±0.76 | 1.01±0.82 | 0.639 |
| Number of treated vessels | 0.95±0.59 | 0.88±0.57 | 0.323 |
| Baseline percentage of stenosis | 83.88±26.19 | 84.75±26.24 | 0.783 |
| Number of implanted stents | 0.93±0.81 | 1.06±0.87 | 0.233 |
| Thrombectomy | 41 (43.2%) | 54 (33.3%) | 0.115 |
| Stent diameter (mm) | 3.31±0.62 | 3.22±0.49 | 0.260 |
| Stent length (mm) | 20.46±10.63 | 20.29±6.32 | 0.983 |
| Bare-metall stent | 44 (56.4%) | 66 (46.8%) | 0.287 |
| Drug-eluting stent | 34 (41.6%) | 75 (53.2%) | 0.287 |
| TIMI flow post-PCI | |||
| TIMI 0 | 7 (7.2%) | 18 (10.7%) | 0.356 |
| TIMI 1 | 0 (0%) | 0 (0%) | |
| TIMI 2 | 0 (0%) | 2 (1.2%) | |
| TIMI 3 | 90 (92.8%) | 149 (88.2%) | |
| Final percentage of stenosis | 10.42±27.27 | 12.78±31.92 | 0.521 |
| Procedure success | 87 (91.6%) | 146 (86.9%) | 0.252 |
| Referred for coronary revascularization surgery | 2 (1.9%) | 9 (4.8%) | 0.198 |
AHA: American Heart Association; CAD: coronary artery disease; PCI: percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction.
Pharmacological treatment at hospital discharge.
| CAD ≤35(n=107) | No CAD ≤35(n=54) | CAD 36–40(n=190) | p-ValueCAD ≤35 vs No CAD ≤35 | p-ValueCAD ≤35 vs CAD 36–40 | |
|---|---|---|---|---|---|
| P2Y12 inhibitors | 92 (90.2%) | 8 (15.1%) | 164 (88.6%) | <0.001 | 0.686 |
| ASA | 102 (99%) | 14 (26.4%) | 180 (97.3%) | <0.001 | 0.324 |
| Anticoagulants | 6 (5.9%) | 2 (3.8%) | 3 (1.7%) | 1.000 | 0.077 |
| Beta-blockers | 79 (77.5%) | 10 (18.9%) | 143 (79.9%) | <0.001 | 0.630 |
| ACE inhibitors | 43 (42.2%) | 6 (11.3%) | 86 (48%) | <0.001 | 0.341 |
| ARBs | 5 (4.9%) | 0 | 6 (3.4%) | 0.171 | 0.535 |
| Calcium channel blockers | 13 (12.7%) | 4 (7.5%) | 15 (8.4%) | 0.429 | 0.240 |
| Statins | 90 (87.4%) | 4 (7.7%) | 161 (89.4%) | <0.001 | 0.598 |
| Diuretics | 7 (6.9%) | 3 (5.7%) | 12 (6.7%) | 1.000 | 0.969 |
| Antidiabetics | 3 (2.9%) | 0 | 2 (1.1%) | 0.551 | 0.358 |
| Antiarrhythmics | 1 (1%) | 1 (1.9%) | 4 (2.2%) | 1.000 | 0.656 |
Data are given as number (percentage). ACE: inhibitors; angiotensin converting enzyme inhibitors; ARBs: angiotensin II receptor blocker; ASA: acetylsalicylic acid; CAD: coronary artery disease.
To compare the groups ≤35 years, cases vs controls, the risk factors that showed a statistically significant relationship with the presence of CAD were smoking (OR 2.49; 95% CI 1.03–6.03; p=0.042) and family history of CAD (OR 6.70; 95% CI 1.46–30.65; p=0.014). Other risk factors such as dyslipidemia, cannabis use lost statistical significance in the multivariate analysis.
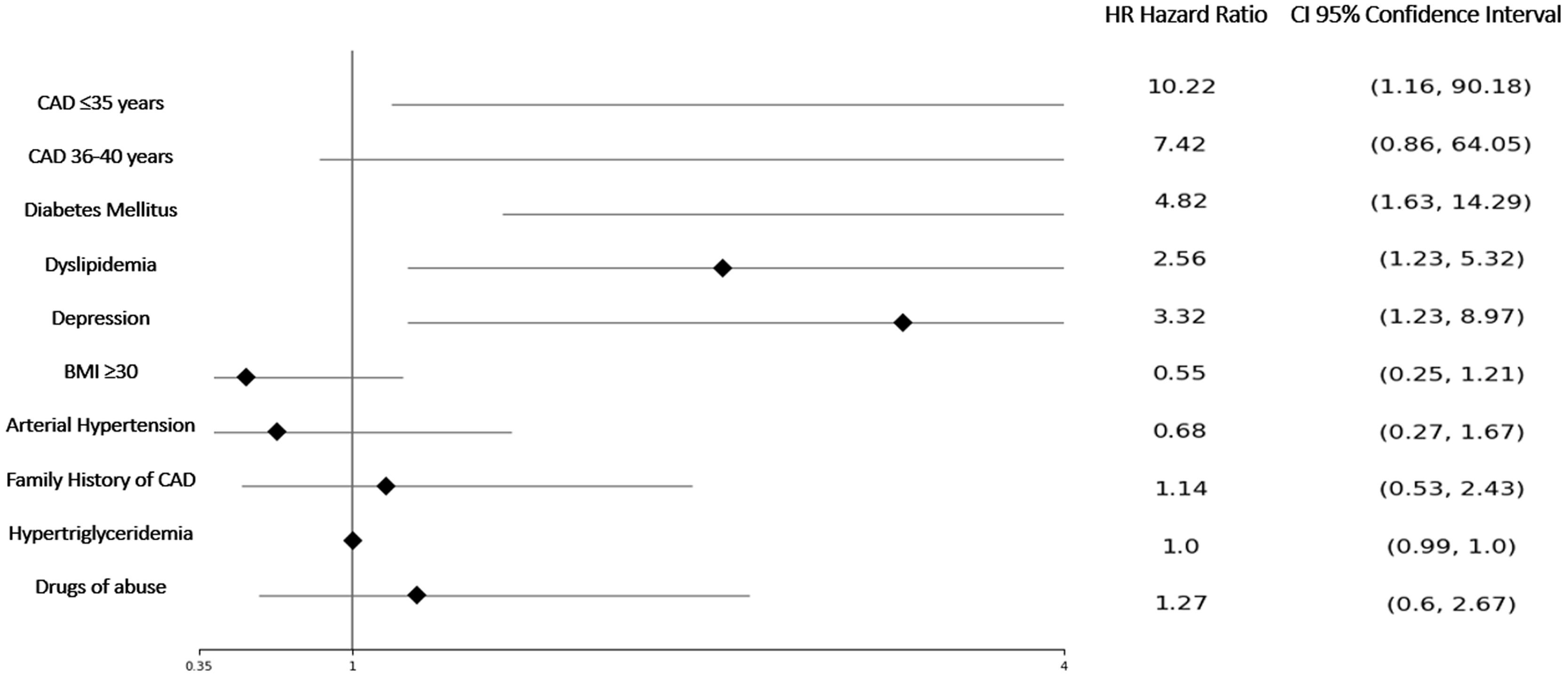
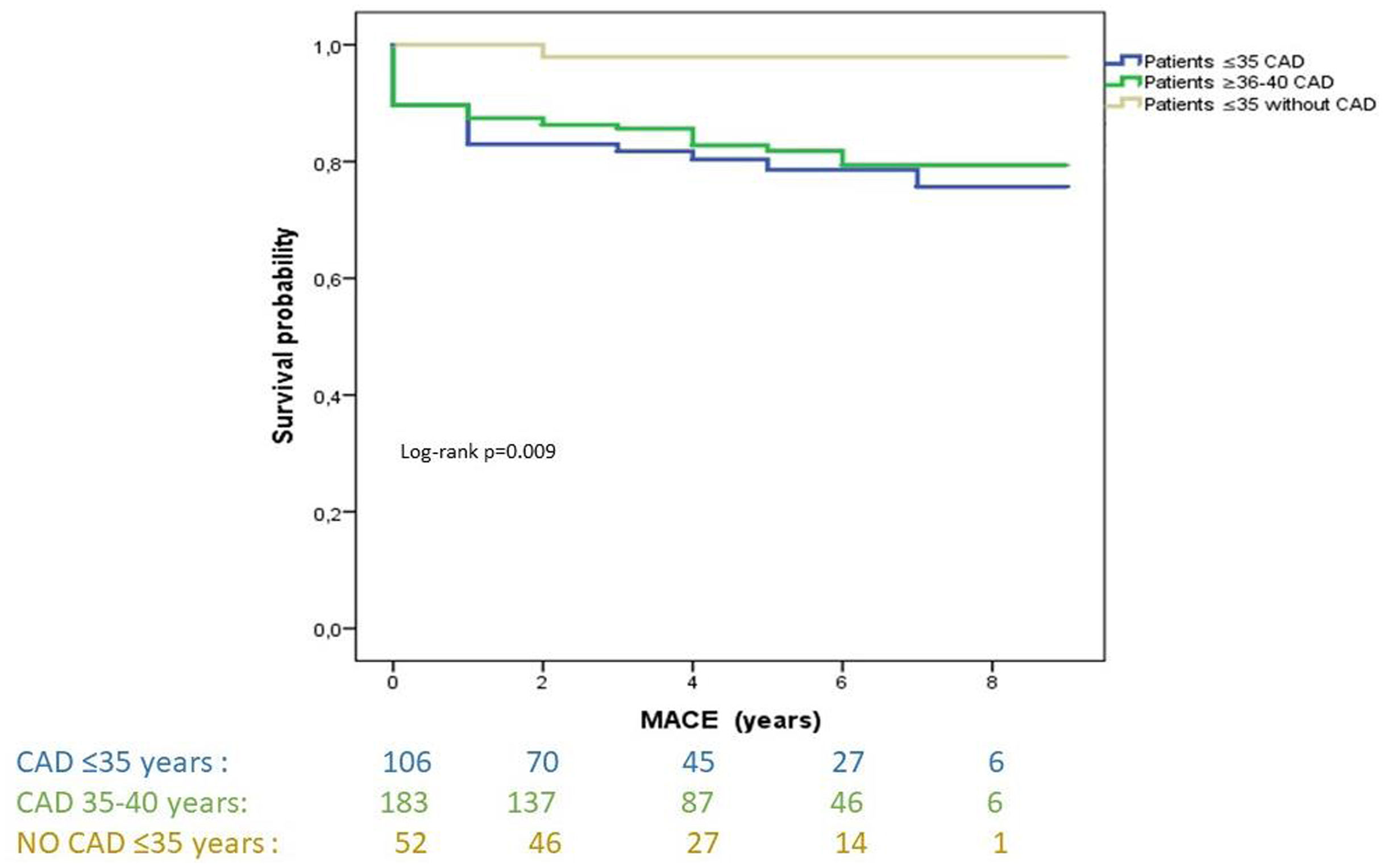
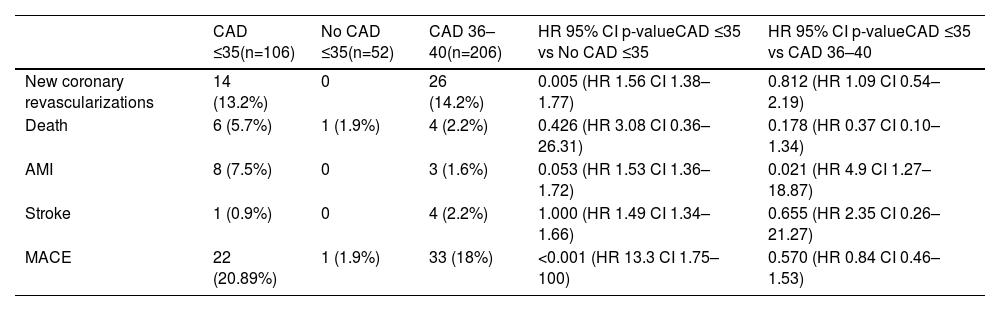
The mean follow-up time in the ≤35 years with CAD (cases) was 4.8±2.4 years, in control A group ≤35 years was 4.87±2.1, and 5.2±2.2 years in the control B group 36–40 years with CAD. During this time, 56 events were detected, all of them listed in Table 5. The group aged ≤35 years with CAD exhibited a 4.5-fold increase in the risk of acute myocardial infarction during follow-up compared to the 36–40 years with CAD group, and more than 1.6-fold increase in the risk of experiencing new coronary revascularization compared to the control group A. Furthermore, both the ≤35 years with CAD group and the 36–40 years with CAD group showed a higher risk of MACE when compared to the control group A (HR 13.3; 95% CI 1.75–100; p<0.001 and HR 7.42; 95% CI 0.86–64.05; p=0.068; respectively). The probability of MACE was associated with being ≤35 years old, having diabetes, dyslipidemia, and depression (Figure 1). Kaplan–Meier curves for MACE-free survival among the three study groups are displayed in Figure 2.
Adverse events in the study period.
| CAD ≤35(n=106) | No CAD ≤35(n=52) | CAD 36–40(n=206) | HR 95% CI p-valueCAD ≤35 vs No CAD ≤35 | HR 95% CI p-valueCAD ≤35 vs CAD 36–40 | |
|---|---|---|---|---|---|
| New coronary revascularizations | 14 (13.2%) | 0 | 26 (14.2%) | 0.005 (HR 1.56 CI 1.38–1.77) | 0.812 (HR 1.09 CI 0.54–2.19) |
| Death | 6 (5.7%) | 1 (1.9%) | 4 (2.2%) | 0.426 (HR 3.08 CI 0.36–26.31) | 0.178 (HR 0.37 CI 0.10–1.34) |
| AMI | 8 (7.5%) | 0 | 3 (1.6%) | 0.053 (HR 1.53 CI 1.36–1.72) | 0.021 (HR 4.9 CI 1.27–18.87) |
| Stroke | 1 (0.9%) | 0 | 4 (2.2%) | 1.000 (HR 1.49 CI 1.34–1.66) | 0.655 (HR 2.35 CI 0.26–21.27) |
| MACE | 22 (20.89%) | 1 (1.9%) | 33 (18%) | <0.001 (HR 13.3 CI 1.75–100) | 0.570 (HR 0.84 CI 0.46–1.53) |
AMI: acute myocardial infarction; CAD: coronary artery disease; CI: confidence interval; MACE: mayor adverse cardiac event.
To the best of our knowledge, this is the first report investigating the risk factors and long-term outcomes in patients aged ≤35 years, compared to two age-matched control groups. The main findings of our study can be summarized as follows: first, only smoking and having a family history of CAD revealed a risk of presentation of premature CAD. Second, patients ≤35 years showed unfavorable long-term prognosis. Third, subjects ≤35 years with a history of diabetes, dyslipidemia and depression have a higher risk of MACE at long-term follow-up.
Patients sought healthcare primarily for chest pain, but younger individuals and those without CAD presented more dyspnea as index symptom. The CAD groups showed single-vessel disease in most of cases, characterized by atherosclerotic plaques with low calcium content and presence of abundant thrombotic material.10 This correlates with the predominant presentation as ACS. Thrombus formation due to plaque erosion is a frequent occurrence in the young population with ACS, predominantly observed on sites of pathological intimal thickening or fibroatheromas.11–13 Furthermore, it is worth noting that over 10% of young patients with CAD received a diagnosis of MINOCA. This finding highlights the importance of considering MINOCA as a potential cause of acute myocardial infarction in a subset of CAD patients and underscores the need for precise diagnostic evaluation and tailored management strategies for this specific population.14,15
The baseline characteristics of the sample reveal a low representation of females, potentially attributed to estrogen protection.16 Additionally, Lorca et al. found that premature ST elevation myocardial infarction was significantly more likely to have chromosome Y.2 Classic risk factors, such as hypertension and diabetes, are present at a relatively low percentage in all three groups, consistent with findings reported by previous authors.17–21 The prevalence of obesity is highest in the 36–40 years group, while the other two groups exhibit similar rates, suggesting a direct correlation of obesity with advancing age. It is noteworthy that the prevalence of dyslipidemia is 33% in the group of patients aged ≤35 years with CAD, while, in the 36–40 years group, it is observed in over half of the population. We found significant variability among the reviewed studies about dyslipidemia prevalence. Kofflard et al., 28 (49%) and Hosseini et al., 51 (47%) present higher percentages compared to the studies by Ruiz et al., 20 (32%) and Lv et al., 74 (21%), which show ratios consistent with our results. This divergence could potentially be attributed to differences in sample sizes, methodologies employed or variations in the populations under study,10,18,19,22 but not exhibiting a direct correlation in this age group. These findings emphasize the potential value of early primary prevention for reducing the occurrence of CAD. Moreover, the consumption of toxic substances, particularly cannabis and cocaine, are markedly higher in our entire study population compared to what is published in the general population.23 Notably, in our group of patients aged ≤35 years with CAD, the prevalence of substance use exceeds 20%.19 A relationship was found between these patients and a family history of CAD and smoking habits, resulting thus in very premature CAD. These results are similar to the findings of other studies.10,14,19,22 They highlight the importance of adequate primary prevention in these patients who are at very high ischemic risk.
Our study results are in line with previous studies.10,19 Kelly et al. found that young patients without standard modifiable risk factors exhibited unfavorable prognoses, accentuating the importance of addressing alternative risk factors,24 particularly those associated with oxidative stress and inflammation.25,26 For other classic risk factors such as dyslipidemia, hypertension, or diabetes, there was no significant relationship in our population. However, the proportion of diabetic and hypertensive patients among very young individuals is very low. Furthermore, the short duration of the influence of these diseases appears to have a limited impact on the manifestation of CAD in young patients. While there is limited evidence comparing such young populations with and without ischemic heart disease, studies have indeed found similar results.19,27,28 The potential role of a family history of CAD in the development of CAD in the young can be attributed to genetic variants and an inherited lifestyle that predisposes people to CAD.29–32 Additionally, tobacco consumption is one of the main triggers of plaque erosion, a mechanism that promotes acute thrombosis.33,34 So, the duration of smoking should not influence the associated risk.
During the follow-up period, there was a significantly higher incidence of reinfarction and MACE in the ≤35 years group, compared to the 36–40 years group and the group of young individuals without CAD.19,22,28 This unexpected finding may be attributed to subclinical familial/genetic factors leading to earlier and more aggressive CAD. Alternatively, poor adherence to lifestyle changes and medical treatment may also contribute to the observed differences. In our study, a significant association was observed between MACE and diabetes, suggesting that metabolic dysfunction may play a role in the acute presentation and progression of premature cardiovascular disease.19,25,35 Also, the results revealed a positive correlation with dyslipidemia, emphasizing the importance of the lipid profile in the progression of cardiovascular diseases.19,25 Our findings highlight a significant relationship between MACE and depression. The study by Vyas et al. compares two cohorts of young patients with depression and their relationship with MACE, revealing a positive association,36 similar to others reviews.37,38 This underscores the need for comprehensive mental health evaluation in patients with premature CAD to improve clinical management and long-term outcomes.38,39 It is important to note that there is a scarcity of published data on the long-term outcomes of these very young patients. Moreover, previous studies investigating the prognosis of ≤35 years old patients are limited and present discordant finding.19,22,24,28 Therefore, our study adds valuable insights to this underexplored area of research. Perhaps leveraging novel advanced computational techniques, such as machine/deep learning, could aid physicians in the decision-making process for the chronic management of this young population.39,40
This study has several limitations. Firstly, it did not encompass an analysis of non-traditional cardiovascular risk factors, such as lipoprotein A, hyperhomocysteinemia, and familial hypercholesterolemia. Additionally, the relationship between prothrombotic diseases and the early onset of ischemic heart disease could not be thoroughly assessed due to insufficient data available for most patients. Other limitations, such as the compliance to medication, blood pressure control and recovery of left ventricular fraction and bias, were not measured or collected for the current model.
Although efforts were made to minimize selection biases, the nature of our study inherently prevents complete elimination of such biases. However, it is important to highlight that our study features a consecutive cohort of patients, yielding the largest sample size reported in the literature for this specific context.
ConclusionIn our study, patients aged ≤35 with CAD exhibited poor long-term prognosis, with a high risk of new revascularization and acute myocardial infarction during follow-up. The presence of diabetes, dyslipidemia, depression, and age <35 years old were identified as factors that increased the risk of MACE at follow-up. To improve outcomes for this group of patients, it is crucial to concentrate efforts on both primary and secondary prevention strategies aimed at preventing recurrences. Focusing on preventive measures can significantly impact the overall prognosis and enhance the quality of life for these individuals.
Ethical approvalTerritorial Research Ethics Committee of Pontevedra-Vigo-Ourense, with registration code 2015/506.
Authors’ contributionsConceptualization: Pablo Juan-Salvadores. Data curation: Pablo Juan-Salvadores, Dahyr Olivas-Medina. Formal analysis: Cesar Veiga, Silvia Campanioni. Funding acquisition: Andrés Íñiguez Romo. Methodology: Pablo Juan-Salvadores, Víctor Alfonso Jiménez Díaz. Project administration: Pablo Juan-Salvadores. Writing – original draft: Pablo Juan-Salvadores, Dahyr Olivas-Medina, Victor Alfonso Jiménez Díaz. Writing – review & editing: Andrés Iñiguez Romo, Francisco Caamaño Isorna, Cesar Veiga, Silvia Campanioni.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors want to thank the Cardiovascular Department staff for their support and help.