Transcatheter aortic valve implantation (TAVI) is an alternative therapeutic approach to patients not considered suitable for surgical aortic valve replacement (SAVR) due to their high operative risk. We sought to assess the impact of TAVI on the profile and operative results of patients with severe aortic stenosis undergoing SAVR.
MethodsA total of 214 patients were included, of whom 103 consecutive patients underwent isolated SAVR in 2005 and 111 in 2009. Patients’ demographic and operative data were collected retrospectively. Operative and one-year mortality and morbidity were analyzed.
ResultsPatients’ mean age was 70 years, and 56% were female. Following the introduction of a TAVI program, patients undergoing conventional surgery were older, with more comorbidities. Overall 30-day and one-year mortality were 2.8% and 7.0%, respectively. After the introduction of TAVI, the observed mortality rate for SAVR decreased, but not significantly (operative mortality: 3.9% before TAVI vs. 1.8% after TAVI, p=NS; one-year mortality: 10% vs. 4.5%, p=NS). Striking differences were observed in morbidity (operative morbidity: 23.3% before TAVI vs. 13.5% after TAVI, p=0.047, and one-year morbidity: 20.4% vs. 9.9%, p=0.032).
ConclusionsSince the introduction of a TAVI program at our center, the number of patients undergoing SAVR has increased, with a slight rise in surgical risk, but without worsening the final operative results. The implementation of a TAVI program has thus had a positive impact on the volume of procedures, patient selection and outcomes in SAVR.
A implantação percutânea de próteses valvulares aórticas (TAVI) é uma alternativa terapêutica na abordagem dos doentes com estenose aórtica severa (EA) inoperáveis. Pretendeu-se avaliar o impacto do programa de TAVI no perfil dos doentes com EA submetidos a cirurgia de substituição valvular aórtica (AVR) isolada e nos resultados obtidos a curto e médio prazo.
MétodosIncluídos 214 doentes consecutivamente operados, dos quais 103 foram operados em 2005 e 111 em 2009, respetivamente, dois anos antes e dois anos após o início da TAVI no nosso centro. Os dados clínicos foram retrospetivamente extraídos. Analisados eventos de morbi-mortalidade no período pós-operatório e ao primeiro ano de seguimento.
ResultadosA idade média dos doentes foi de 70 anos e a maioria do sexo feminino (56%). Na era pós-TAVI, foram operados doentes tendencialmente mais velhos e com mais comorbilidades. As mortalidades operatórias ao primeiro ano observadas foram de 2,8 e 7%. Após a introdução da TAVI, a mortalidade diminuiu, sem atingir significância estatística (mortalidade operatória: 3,9% pré-TAVI versus 1,8% pós-TAVI, p = ns e mortalidade no primeiro ano: 10 versus 4,5%, p = ns) e os indicadores de morbilidade melhoraram significativamente (morbilidade operatória: 23,3% pré-TAVI versus 13,5% pós-TAVI, p = 0,047 e morbilidade ao primeiro ano: 20,4 versus 9,9%, p = 0,032).
ConclusõesDesde o início do programa de TAVI no nosso centro, o volume de AVR aumentou e foram operados doentes com pior perfil de risco, sem prejuízo dos indicadores de morbi-mortalidade. Teve assim um impacto positivo no volume de cirurgias realizadas, na seleção dos doentes operados e nos resultados obtidos.
Aortic valve stenosis is increasingly common in developed countries as a result of ageing populations.1 Patients with this condition are at higher risk of sudden death and, when symptomatic, mean survival is only 2–3 years.2 Surgical aortic valve replacement (SAVR) is the gold standard treatment for symptomatic patients with severe aortic stenosis, and has been shown to improve symptoms and survival.3,4 In recent years, technological advances and overall improvements in health care have allowed older patients with higher surgical risk to be operated with lower operative mortality.
Notwithstanding improved mortality rates, many patients are still considered inoperable due to prohibitive risk,5 and a significant percentage of patients are not even referred to surgical centers. Studies have estimated that a third of patients with severe aortic stenosis are not operated.6
Transcatheter aortic valve implantation (TAVI) was developed as an alternative to conventional aortic valve surgery in high-risk patients, and has shown very promising results.7
A TAVI program was introduced in our tertiary center in August 2007 to treat patients with severe aortic stenosis who were deemed unsuitable for conventional cardiac surgery.
We sought to assess the impact of this program on the volume of SAVR procedures performed, and on the profile of operated patients and outcomes in terms of operative and one-year morbidity and mortality.
MethodsStudy population and data collectionThis was a single-center retrospective study of consecutive patients who underwent isolated SAVR for severe aortic stenosis (functional area <1.0 cm2 or mean gradient >40 mmHg) in 2005 and 2009 (respectively two years before and two years after implementation of the TAVI program).
Patients’ demographic, clinical and echocardiographic data were collected from medical and hospital records. Surgical risk was estimated for each patient based on the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE), using the online calculator. Surgical procedures were classified as elective, urgent or emergency. Elective patients were scheduled for surgery and admitted accordingly, while non-elective patients were hospitalized as a result of clinical instability and/or life-threatening situations (such as congestive heart failure [HF], low cardiac output and cardiogenic shock) and were operated either immediately (emergency surgery) or during the same hospitalization (urgent surgery).
Operative mortality was defined as all-cause death within 30 days of surgery (including during hospital stay). Operative morbidity was defined as the occurrence during the post-operative period of any of the following: death, prolonged (>24 hours) invasive mechanical ventilation, severe arrhythmias (ventricular fibrillation or recurrent ventricular tachycardia, new-onset atrial flutter or fibrillation, or bradyarrhythmia requiring pacemaker), myocardial infarction (elevation of serum troponin I to levels five times higher than pre-surgery values), severe HF (NYHA class III-IV), moderate to large pericardial or pleural effusion, pneumonia, pyelonephritis, acute renal failure (serum creatinine >2.0 mg/dl or twice pre-surgery values or need for de novo dialysis), stroke, major bleeding (resulting in hemodynamic compromise or need for transfusion of more than 2–3 units of red cells or urgent surgical intervention), cardiac reoperation, mediastinitis or surgical wound infection.
One-year follow-up, achieved in 94% of patients, was based on data from medical consultations, urgent hospitalizations and telephone interviews. The following events were recorded: all-cause and cardiac death, myocardial infarction, stroke, rehospitalization for cardiac cause, cardiac re-operation, and thromboembolic and bleeding complications.
Statistical analysisCategorical variables are presented as percentages and frequencies and were compared using the chi-square test. Continuous variables are expressed as means ± standard deviation and were compared using the Mann-Whitney test. A value of p<0.05 was considered statistically significant. Predicted mortality was calculated for each patient using the logistic EuroSCORE and compared to observed mortality. The discriminatory power of this risk algorithm was assessed through determination of the area under the curve (AUC) and considered to be significant when >0.7. Survival analysis was based on the Kaplan-Meier method. Univariate logistic regression analysis was used to determine predictors of operative mortality (p<0.05), which were then included in the multivariate model (backward Wald logistic regression). All statistical analyses were performed using SPSS for Windows version 17 (SPSS Inc.).
ResultsVolume of surgical proceduresDuring the two years analyzed in the study, 214 patients underwent isolated SAVR; of these 103 were operated two years before implementation of the TAVI program and 111 two years after, an increase of 8% in the volume of surgical procedures.
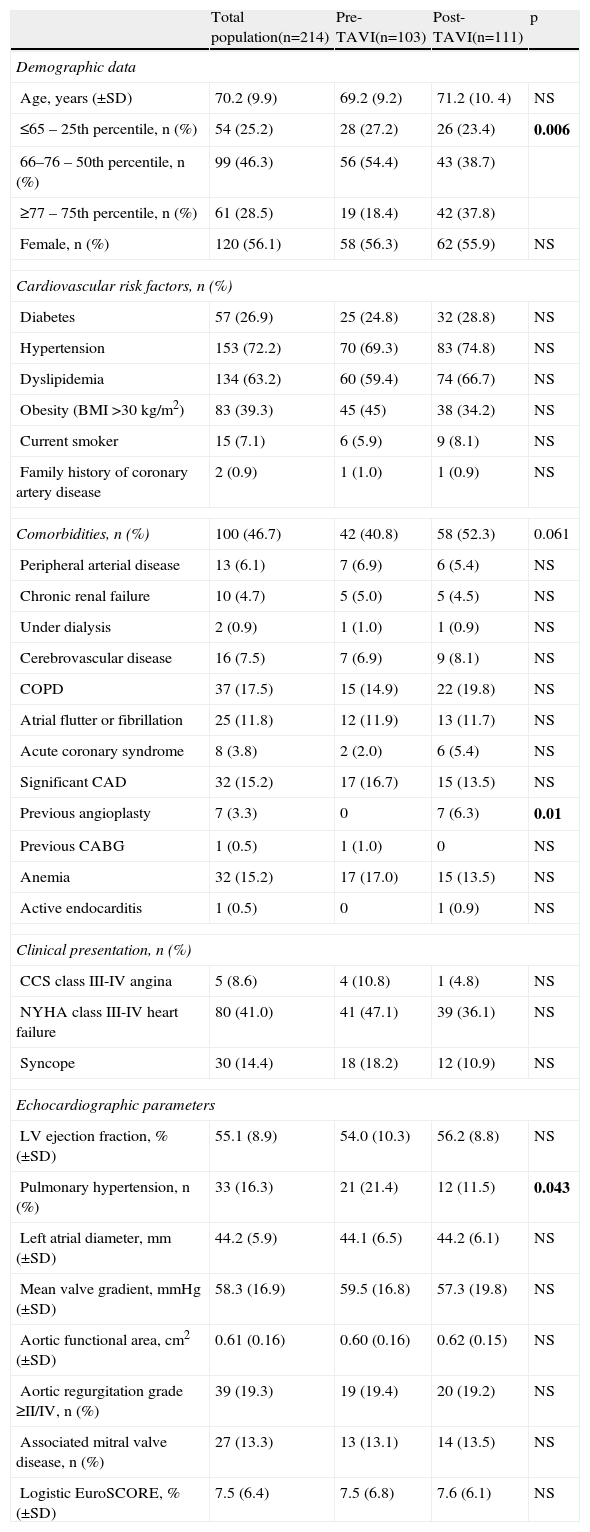
Profile of operated patientsThe mean age of the population was 70±9.9 years (range 35–86), and most patients were women (56.1%). The most prevalent cardiovascular risk factors were hypertension (72%), dyslipidemia (63%), obesity (39%) and diabetes (27%). Almost half (47%) had at least one comorbidity, the most common being chronic obstructive pulmonary disease (COPD) (18%), significant coronary artery disease (15%), anemia (15%) and atrial fibrillation or flutter (12%). Of the 32 patients with significant coronary artery disease, eight had previously undergone percutaneous (n=7) or surgical (n=1) revascularization; one underwent single-vessel angioplasty in the post-operative period. The remainder had not been revascularized for the following reasons: absence of ischemia and/or documented myocardial viability, distal lesions, lesion in a minor branch, or a vascular bed distal to a minor lesion. Half the patients had significant symptoms at the time of surgery (41% with NYHA class III-IV HF, and 9% with CCS class III-IV angina). In hemodynamic terms, mean aortic functional area was 0.6±0.16 cm2 (range 0.2–1.2 cm2) and mean valve gradient was 60±16.9 mmHg (range 20–130 mmHg). Most patients had preserved ejection fraction (mean 55±8.9%, range 20–79%). Around 13% of patients (n=27) had associated mitral valve disease, including four with grade II/IV mitral stenosis, 22 with grade II/IV mitral regurgitation and one with grade III/IV, predominantly functional. Echocardiographic signs suggestive of pulmonary hypertension (pulmonary artery systolic pressure >40 mmHg higher than central venous pressure) were observed in 16% of patients. The mean logistic EuroSCORE was 7.5±6.4% (range 1.5–44.6), reflecting a population with high surgical risk (EuroSCORE >6).
Patients operated in the post-TAVI period were older, with a higher proportion above the 75th percentile for age (18.4% of patients operated pre-TAVI were aged ≥77 years vs. 37.8% post-TAVI, p=0.006). They also tended to have more comorbidities (40.8% pre-TAVI vs. 52.3% post-TAVI, p=0.061), particularly COPD (14.9% vs. 19.8%, p=NS), acute coronary syndrome (2.0% vs. 5.4%, p=NS) and previous coronary angioplasty (0% vs. 6.3%, p=NS). A lower proportion of patients operated in the post-TAVI period had echocardiographic signs of pulmonary hypertension (21.4% vs. 11.5%, p=0.043). The two groups are compared in Table 1.
Characteristics of the total population and comparison between patients operated before and after implementation of the TAVI program.
| Total population(n=214) | Pre-TAVI(n=103) | Post-TAVI(n=111) | p | |
| Demographic data | ||||
| Age, years (±SD) | 70.2 (9.9) | 69.2 (9.2) | 71.2 (10. 4) | NS |
| ≤65 – 25th percentile, n (%) | 54 (25.2) | 28 (27.2) | 26 (23.4) | 0.006 |
| 66–76 – 50th percentile, n (%) | 99 (46.3) | 56 (54.4) | 43 (38.7) | |
| ≥77 – 75th percentile, n (%) | 61 (28.5) | 19 (18.4) | 42 (37.8) | |
| Female, n (%) | 120 (56.1) | 58 (56.3) | 62 (55.9) | NS |
| Cardiovascular risk factors, n (%) | ||||
| Diabetes | 57 (26.9) | 25 (24.8) | 32 (28.8) | NS |
| Hypertension | 153 (72.2) | 70 (69.3) | 83 (74.8) | NS |
| Dyslipidemia | 134 (63.2) | 60 (59.4) | 74 (66.7) | NS |
| Obesity (BMI >30 kg/m2) | 83 (39.3) | 45 (45) | 38 (34.2) | NS |
| Current smoker | 15 (7.1) | 6 (5.9) | 9 (8.1) | NS |
| Family history of coronary artery disease | 2 (0.9) | 1 (1.0) | 1 (0.9) | NS |
| Comorbidities, n (%) | 100 (46.7) | 42 (40.8) | 58 (52.3) | 0.061 |
| Peripheral arterial disease | 13 (6.1) | 7 (6.9) | 6 (5.4) | NS |
| Chronic renal failure | 10 (4.7) | 5 (5.0) | 5 (4.5) | NS |
| Under dialysis | 2 (0.9) | 1 (1.0) | 1 (0.9) | NS |
| Cerebrovascular disease | 16 (7.5) | 7 (6.9) | 9 (8.1) | NS |
| COPD | 37 (17.5) | 15 (14.9) | 22 (19.8) | NS |
| Atrial flutter or fibrillation | 25 (11.8) | 12 (11.9) | 13 (11.7) | NS |
| Acute coronary syndrome | 8 (3.8) | 2 (2.0) | 6 (5.4) | NS |
| Significant CAD | 32 (15.2) | 17 (16.7) | 15 (13.5) | NS |
| Previous angioplasty | 7 (3.3) | 0 | 7 (6.3) | 0.01 |
| Previous CABG | 1 (0.5) | 1 (1.0) | 0 | NS |
| Anemia | 32 (15.2) | 17 (17.0) | 15 (13.5) | NS |
| Active endocarditis | 1 (0.5) | 0 | 1 (0.9) | NS |
| Clinical presentation, n (%) | ||||
| CCS class III-IV angina | 5 (8.6) | 4 (10.8) | 1 (4.8) | NS |
| NYHA class III-IV heart failure | 80 (41.0) | 41 (47.1) | 39 (36.1) | NS |
| Syncope | 30 (14.4) | 18 (18.2) | 12 (10.9) | NS |
| Echocardiographic parameters | ||||
| LV ejection fraction, % (±SD) | 55.1 (8.9) | 54.0 (10.3) | 56.2 (8.8) | NS |
| Pulmonary hypertension, n (%) | 33 (16.3) | 21 (21.4) | 12 (11.5) | 0.043 |
| Left atrial diameter, mm (±SD) | 44.2 (5.9) | 44.1 (6.5) | 44.2 (6.1) | NS |
| Mean valve gradient, mmHg (±SD) | 58.3 (16.9) | 59.5 (16.8) | 57.3 (19.8) | NS |
| Aortic functional area, cm2 (±SD) | 0.61 (0.16) | 0.60 (0.16) | 0.62 (0.15) | NS |
| Aortic regurgitation grade ≥II/IV, n (%) | 39 (19.3) | 19 (19.4) | 20 (19.2) | NS |
| Associated mitral valve disease, n (%) | 27 (13.3) | 13 (13.1) | 14 (13.5) | NS |
| Logistic EuroSCORE, % (±SD) | 7.5 (6.4) | 7.5 (6.8) | 7.6 (6.1) | NS |
Associated mitral valve disease: grade ≥II/IV mitral regurgitation or stenosis; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; Peripheral arterial disease: intermittent claudication, >50% carotid stenosis or previous vascular surgery; Pulmonary hypertension: pulmonary artery systolic pressure >40 mmHg higher than central venous pressure; Significant coronary artery disease: ≥70% stenosis (or ≥50% in the left main artery). Bold values denote statistically significant values (<0.05).
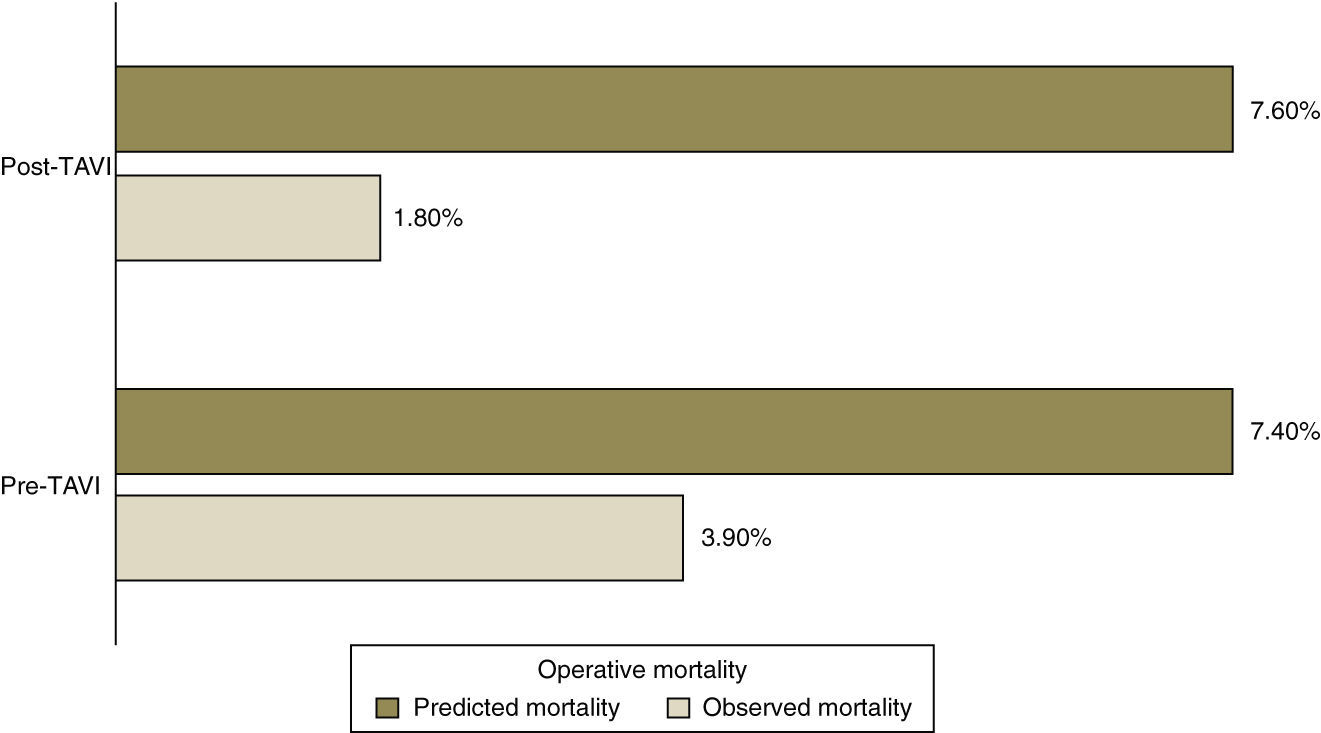
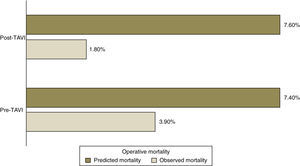
Predicted mortality based on the logistic EuroSCORE was similar for both groups (7.4% pre-TAVI vs. 7.6% post-TAVI, p=NS), but far higher than observed operative mortality (3.9% vs. 1.8%) (Figure 1).
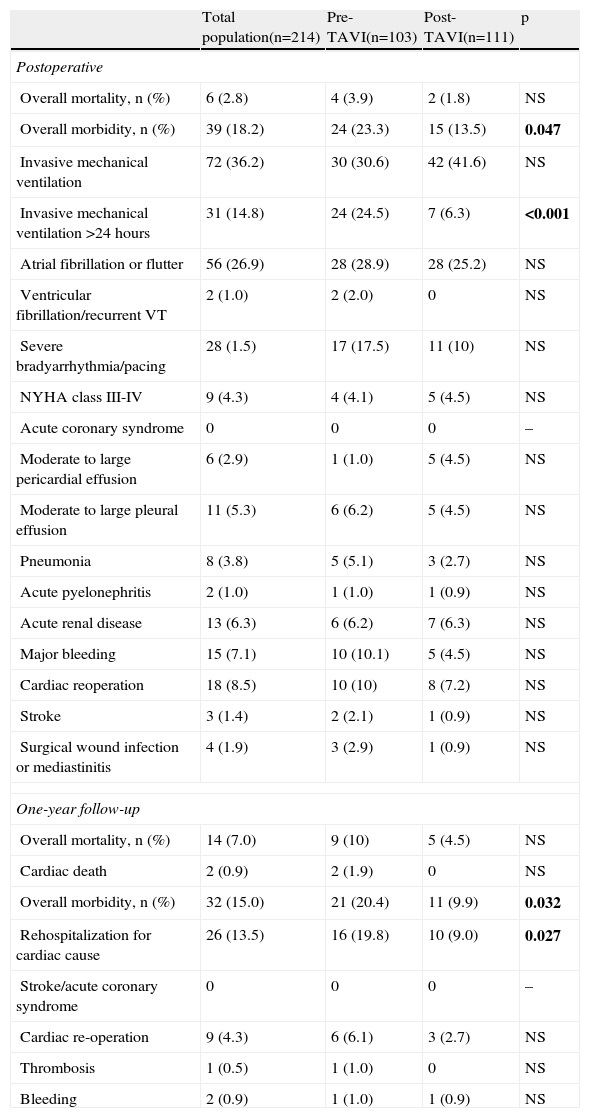
Operative and one-year mortality rates in the total population were 2.8% (n=6) and 7% (n=14), respectively. Causes of death in the post-operative period were septic shock (n=3), refractory HF (n=2) and unknown cause (n=1). Only two of the 14 deaths during one-year follow-up were attributed to cardiac cause.
Observed operative and one-year mortality were lower after implementation of TAVI, but not significantly (3.9% vs. 1.8% and 10% vs. 4.5%, respectively).
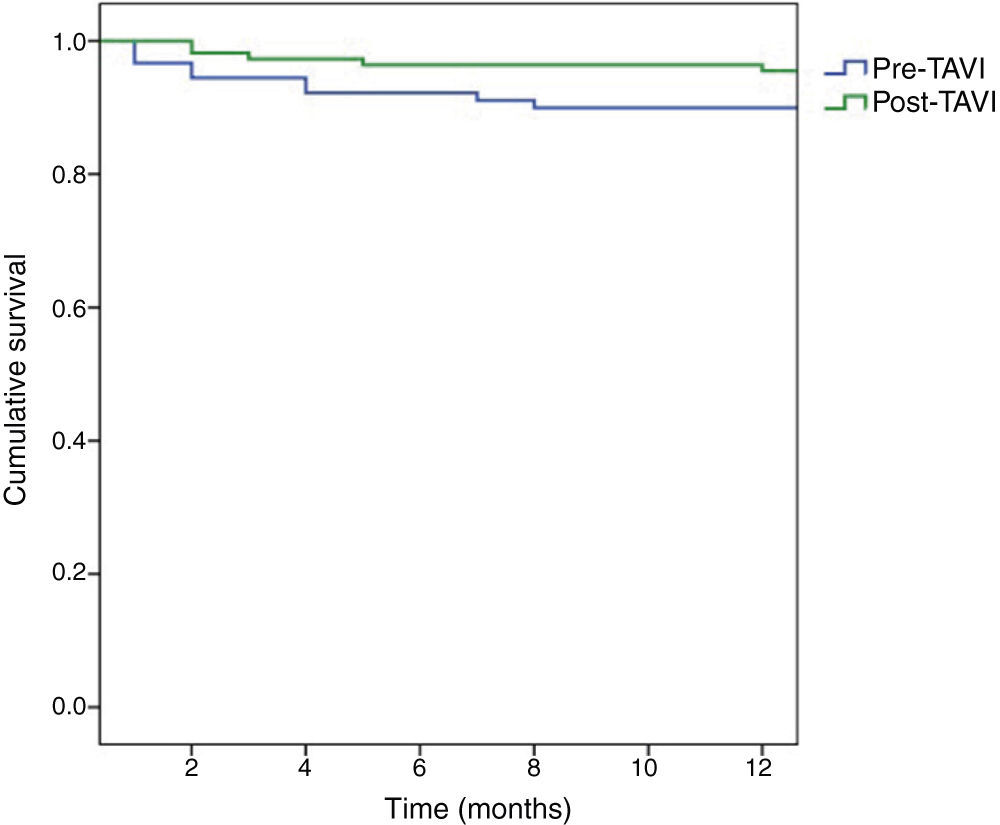
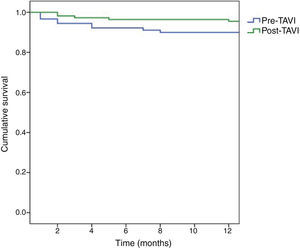
Kaplan-Meier survival curves are shown in Figure 2. At one-year follow-up, survival among patients operated in the pre-TAVI period was 89.9%, with a higher survival rate among those operated in the post-TAVI period (95.5%), but without statistical significance (log-rank test p=0.126).
Short- and medium-term morbiditySignificant differences were found in morbidity between the two groups (Table 2), with more complications in patients operated in the pre-TAVI era, both in the post-operative period (23.3% pre-TAVI vs. 13.5% post-TAVI, p=0.047) and at one year (20.4% vs. 9.9%, p=0.032).
Operative and one-year morbidity and mortality of the total population and comparison between the two groups.
| Total population(n=214) | Pre-TAVI(n=103) | Post-TAVI(n=111) | p | |
| Postoperative | ||||
| Overall mortality, n (%) | 6 (2.8) | 4 (3.9) | 2 (1.8) | NS |
| Overall morbidity, n (%) | 39 (18.2) | 24 (23.3) | 15 (13.5) | 0.047 |
| Invasive mechanical ventilation | 72 (36.2) | 30 (30.6) | 42 (41.6) | NS |
| Invasive mechanical ventilation >24 hours | 31 (14.8) | 24 (24.5) | 7 (6.3) | <0.001 |
| Atrial fibrillation or flutter | 56 (26.9) | 28 (28.9) | 28 (25.2) | NS |
| Ventricular fibrillation/recurrent VT | 2 (1.0) | 2 (2.0) | 0 | NS |
| Severe bradyarrhythmia/pacing | 28 (1.5) | 17 (17.5) | 11 (10) | NS |
| NYHA class III-IV | 9 (4.3) | 4 (4.1) | 5 (4.5) | NS |
| Acute coronary syndrome | 0 | 0 | 0 | – |
| Moderate to large pericardial effusion | 6 (2.9) | 1 (1.0) | 5 (4.5) | NS |
| Moderate to large pleural effusion | 11 (5.3) | 6 (6.2) | 5 (4.5) | NS |
| Pneumonia | 8 (3.8) | 5 (5.1) | 3 (2.7) | NS |
| Acute pyelonephritis | 2 (1.0) | 1 (1.0) | 1 (0.9) | NS |
| Acute renal disease | 13 (6.3) | 6 (6.2) | 7 (6.3) | NS |
| Major bleeding | 15 (7.1) | 10 (10.1) | 5 (4.5) | NS |
| Cardiac reoperation | 18 (8.5) | 10 (10) | 8 (7.2) | NS |
| Stroke | 3 (1.4) | 2 (2.1) | 1 (0.9) | NS |
| Surgical wound infection or mediastinitis | 4 (1.9) | 3 (2.9) | 1 (0.9) | NS |
| One-year follow-up | ||||
| Overall mortality, n (%) | 14 (7.0) | 9 (10) | 5 (4.5) | NS |
| Cardiac death | 2 (0.9) | 2 (1.9) | 0 | NS |
| Overall morbidity, n (%) | 32 (15.0) | 21 (20.4) | 11 (9.9) | 0.032 |
| Rehospitalization for cardiac cause | 26 (13.5) | 16 (19.8) | 10 (9.0) | 0.027 |
| Stroke/acute coronary syndrome | 0 | 0 | 0 | – |
| Cardiac re-operation | 9 (4.3) | 6 (6.1) | 3 (2.7) | NS |
| Thrombosis | 1 (0.5) | 1 (1.0) | 0 | NS |
| Bleeding | 2 (0.9) | 1 (1.0) | 1 (0.9) | NS |
NYHA: New York Heart Association; VT: ventricular tachycardia. Bold values denote statistically significant values (<0.05).
Patients who underwent SAVR in 2005 had more postoperative complications, particularly need for prolonged invasive mechanical ventilation (24.5% pre-TAVI vs. 6.3% post-TAVI, p<0.001), severe bradyarrhythmias (17.5% vs. 10%; p=NS) and major bleeding (10.1% vs. 4.5%, p=NS). This trend continued during one-year follow-up, with a greater number of rehospitalizations for cardiac cause (19.8% vs. 9.0%, p=0.027).
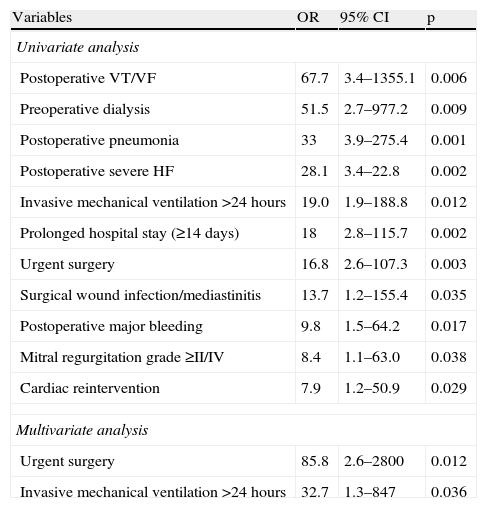
Predictors of operative mortalityOn univariate regression analysis, the following were predictors of operative mortality: history of dialysis (odds ratio [OR] 51.5, 95% confidence interval [CI] 2.7–977.2, p=0.009); significant mitral regurgitation (OR 8.4, 95% CI 1.1–63.0, p=0.038); urgent cardiac surgery (OR 16.8, 95% CI 2.6–107.3, p=0.003); prolonged invasive mechanical ventilation (OR 19.0, 95% CI 1.9–188.8, p=0.012); post-operative ventricular fibrillation or recurrent ventricular tachycardia (OR 67.7, 95% CI 3.4–1355.1, p=0.006), severe HF (OR=28.1, 95% CI 3.4–22.8, p=0.002), pneumonia (OR 33, 95% CI 3.9–275.4, p=0.001), surgical wound infection or mediastinitis (OR 13.7, 95% CI 1.2–155.4, p=0.035), major bleeding (OR 9.8, 95% CI 1.5–64.2, p=0.017), cardiac re-intervention (OR 7.9, 95% CI 1.2–50.9, p=0.029); and prolonged hospital stay (≥14 days) (OR 18, 95% CI 2.8–115.7, p=0.002) (Table 3).
Predictors of operative mortality by univariate and multivariate analysis.
| Variables | OR | 95% CI | p |
| Univariate analysis | |||
| Postoperative VT/VF | 67.7 | 3.4–1355.1 | 0.006 |
| Preoperative dialysis | 51.5 | 2.7–977.2 | 0.009 |
| Postoperative pneumonia | 33 | 3.9–275.4 | 0.001 |
| Postoperative severe HF | 28.1 | 3.4–22.8 | 0.002 |
| Invasive mechanical ventilation >24 hours | 19.0 | 1.9–188.8 | 0.012 |
| Prolonged hospital stay (≥14 days) | 18 | 2.8–115.7 | 0.002 |
| Urgent surgery | 16.8 | 2.6–107.3 | 0.003 |
| Surgical wound infection/mediastinitis | 13.7 | 1.2–155.4 | 0.035 |
| Postoperative major bleeding | 9.8 | 1.5–64.2 | 0.017 |
| Mitral regurgitation grade ≥II/IV | 8.4 | 1.1–63.0 | 0.038 |
| Cardiac reintervention | 7.9 | 1.2–50.9 | 0.029 |
| Multivariate analysis | |||
| Urgent surgery | 85.8 | 2.6–2800 | 0.012 |
| Invasive mechanical ventilation >24 hours | 32.7 | 1.3–847 | 0.036 |
CI: confidence interval; HF: heart failure; OR: odds ratio; VT/VF: recurrent ventricular tachycardia/ventricular fibrillation.
In multivariate analysis, urgent cardiac surgery (86-fold greater risk of death, 95% CI 2.6–2800, p=0.012) and need for prolonged invasive mechanical ventilation (33-fold greater risk of death, 95% CI 1.3–847, p=0.036) were independent predictors of operative mortality (Table 3).
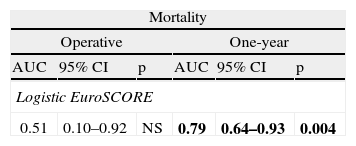
Accuracy of EuroSCORE in predicting surgical riskThere are various algorithms for preoperative risk stratification of cardiac surgery, one of the most commonly used being the logistic EuroSCORE. We analyzed the discriminatory power of this algorithm for operative and one-year mortality, as presented in Table 4.
Discriminatory power of the logistic EuroSCORE for operative and one-year mortality.
| Mortality | |||||
| Operative | One-year | ||||
| AUC | 95% CI | p | AUC | 95% CI | p |
| Logistic EuroSCORE | |||||
| 0.51 | 0.10–0.92 | NS | 0.79 | 0.64–0.93 | 0.004 |
AUC: area under the curve, considered significant when >0.7; CI: confidence interval. Bold values denote statistically significant values (<0.05).
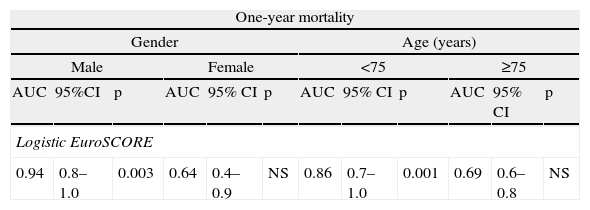
The logistic EuroSCORE did not show discriminatory power for operative mortality (AUC 0.51). It showed satisfactory predictive value for one-year mortality (AUC 0.79, 95% CI 0.64–0.93, p=0.004), particularly in male patients (AUC 0.94, 95% CI 0.8–1.0, p=0.003) and in those aged under 75 (AUC 0.86, 95% CI 0.7–1.0, p=0.001) (Table 5).
Discriminatory power of the logistic EuroSCORE for one-year mortality by gender and age-group.
| One-year mortality | |||||||||||
| Gender | Age (years) | ||||||||||
| Male | Female | <75 | ≥75 | ||||||||
| AUC | 95%CI | p | AUC | 95% CI | p | AUC | 95% CI | p | AUC | 95% CI | p |
| Logistic EuroSCORE | |||||||||||
| 0.94 | 0.8–1.0 | 0.003 | 0.64 | 0.4–0.9 | NS | 0.86 | 0.7–1.0 | 0.001 | 0.69 | 0.6–0.8 | NS |
AUC: area under the curve, considered significant when >0.7; CI: confidence interval; NS: non-significant.
The implementation of a TAVI program in our center was accompanied by an increase in the number of patients undergoing SAVR, for which there are two main reasons.
Firstly, the advent of new technologies and alternative treatments has focused attention on the disease and increased awareness among health professionals, resulting in a greater number of patient referrals.9 Many patients with severe aortic stenosis who are considered to be at high surgical risk are not even referred to surgical centers. However, our center was a pioneer in implementing a TAVI program in the northern and central regions of the country, which enlarged its referral area.
Secondly, with the introduction of percutaneous treatment, management of patients with severe aortic stenosis became a multidisciplinary process.9 In our center, all patients referred for TAVI are thoroughly assessed by a multidisciplinary heart team, which includes specialists in cardiology, cardiothoracic surgery, anesthesiology and vascular surgery. All patients with severe aortic stenosis are initially considered for cardiac surgery and only become candidates for TAVI if their surgical risk is too high. This means that some patients initially referred for percutaneous treatment will in fact be accepted for SAVR.
Following the introduction of the TAVI program, patients undergoing SAVR in our center had markedly higher surgical risk (older age and with more comorbidities) and were at an earlier stage of the disease, before the occurrence of significant repercussions in pulmonary vascular resistance. It can thus be assumed that patients are being referred earlier, the result of greater knowledge of the natural history of the disease and the advantages of prompt treatment, together with improved assessment of disease severity by a variety of more advanced diagnostic exams.
The observed operative mortality in our population was similar to that reported in other studies,5 and was related to worse presurgical status (associated mitral valve disease and regular dialysis) and to indicators of unfavorable prognosis (urgent surgery, certain postoperative complications and need for prolonged hospitalization).
The logistic EuroSCORE did not accurately predict operative mortality in our population, which confirms its tendency to overestimate risk in aortic valve surgery.8 In practical terms, it is of limited use for stratifying preoperative risk and identifying patients at high surgical risk. Nevertheless, as demonstrated in other studies,10 it accurately predicted one-year mortality. In practice, clinical judgement should prevail in therapeutic decisions, taking account of risk estimates and other patient characteristics not included in the algorithms, as well as of the results and experience of the center.11
The lower short- and medium-term morbidity found in our study appears to be mainly related to marked improvements in the care provided and to advances in medical and surgical technologies and in complementary exams.
Recent years have seen very encouraging developments in the management of severe aortic stenosis, a clinical entity that is becoming increasingly prevalent. Patients are diagnosed and referred earlier, undergo multidisciplinary assessment based on a range of reliable complementary exams and receive the treatment most appropriate to their clinical condition, and are treated and followed-up by dedicated, skilled and experienced specialists.
ConclusionsSince the introduction of a TAVI program at our center, the number of patients undergoing SAVR has increased, albeit with a higher predicted surgical risk. This increase has been accompanied by a non-significant reduction in overall mortality and a significant reduction in short- and medium-term morbidity. The implementation of the TAVI program has thus had a positive impact on the volume of procedures, patient selection and outcomes in SAVR.
Ethical disclosuresProtection of human and animalThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Pereira E, Silva G, Caeiro D, et al. Cirurgia cardíaca na estenose aórtica severa: o que mudou com o advento do tratamento percutâneo? Rev Port Cardiol. 2013;32:749–756.