A patient with a dual chamber pacemaker was admitted to the emergency room after out-of-hospital cardioversion for syncopal sustained monomorphic ventricular tachycardia. Device interrogation revealed an abnormally timed ventricular spike after a ventricular premature beat at the beginning of the event, caused by a pacemaker algorithm designed to avoid atrial fibrillation, non-competitive atrial pacing. Despite the absence of significant coronary lesions, in the setting of a vulnerable substrate – a hypokinetic and hyperechogenic region of ventricular myocardium – an upgrade to a dual-chamber implantable cardioverter-defibrillator was performed, and substrate ablation was planned.
Doente com pacemaker de dupla câmara é admitido no Serviço de Urgência após uma cardioversão fora do hospital por episódio sincopal secundário a taquicardia ventricular monomórfica mantida. A interrogação do dispositivo revelou um spike ventricular inapropriado após uma extrassístole ventricular no início do evento, provocado por um algoritmo de pacemaker desenhado para evitar fibrilhação auricular – o non-competitive atrial pacing (NCAP). Apesar da ausência de lesões coronárias significativas, no contexto de substrato vulnerável – uma região hipocinética e hiperecogénica de miocárdio ventricular – upgrade para cardiodesfibrilhador implantável (CDI) bicamaral foi realizado e ablação de substrato planeada.
A 72-year-old male with sinus node disease and paroxysmal atrial fibrillation (AF) had had a Medtronic Adapta® DDDR pacemaker (Minneapolis, MN, USA) implanted eight years before. No history of acute coronary syndromes or heart failure was reported by the patient or in previous medical records. He was taking amiodarone 200 mg/day to prevent AF relapses.
After a brief period of swimming at the beach, he began to suffer palpitations with progressive symptoms of hemodynamic deterioration, leading to syncope. He was promptly assisted by a paramedical team, and monomorphic ventricular tachycardia (VT), with a cycle length of 280 ms, left bundle branch block morphology, superior axis and positive QRS in all precordial leads were documented (Figure 1). Electrical cardioversion was delivered, and, after return to sinus rhythm, no repolarization abnormalities were detected.
After admission to the hospital, urgent coronary angiography showed normal epicardial coronary arteries and cardiac biomarkers levels were not significantly raised. A detailed transthoracic echocardiogram was performed showing ejection fraction of 46% and hypokinesia and slight hyperechogenicity of the mid and basal portions of the posterior and inferior walls. Cardiac magnetic resonance imaging (MRI) was not performed due to the presence of a non-MRI compatible device.
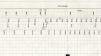
The tachycardia episode was retrieved from device memory, which enabled the detailed sequence of events that started the arrhythmia to be observed. The episode began with a ventricular premature beat (VPB) (Figure 2a) with retrograde (ventriculoatrial) conduction (Figure 2b). Due to the VPB and the post-ventricular atrial refractory period (PVARP) extension algorithm being programmed on, the retrograde atrial activity was sensed as a refractory atrial event. As the patient had suffered paroxysmal AF episodes, the device's non-competitive atrial pacing algorithm (NCAP)1 (Medtronic, Minneapolis, MN, USA) had also been switched on. This algorithm is designed to avoid pacing in the atrium in the vulnerable period after a premature beat, which could potentially induce AF. So, when an atrial refractory event is sensed, a 300-ms window is opened, during which no atrial pacing is delivered (Figure 2c). Atrial pacing then resumes (Figure 2d). Concomitantly, to avoid excessive variation in ventricular cycles due to the NCAP delay, after the atrial stimulus, although a paced atrioventricular interval begins at the programmed value, the ventricular stimulus is delivered earlier, nominally 30 ms after the atrial stimulus (Figure 2e). In this particular case, the combination of these two algorithms induced a short-long-short sequence, which together with a probable vulnerable substrate induced sustained monomorphic VT (Figure 2f). Considering hemodynamically unstable VT started by a properly timed extra stimulus provided by the device, and the clues favoring the existence of a vulnerable myocardial substrate, with an electrocardiogram showing a ventricular tachycardia whose morphology suggests originated in the hypokinetic region identified in the echocardiogram, an upgrade to a dual-chamber implantable cardioverter-defibrillator was performed and VT ablation was planned.
Markers retrieved from the pacemaker memory detailing the sequence of events inducing ventricular tachycardia (VT): ventricular premature beat (a) with retrograde (ventriculoatrial) conduction (b); since an atrial refractory event is sensed a window is opened during which no atrial pacing is delivered (c); atrial pacing then resumes (d), and a ventricular stimulus is delivered earlier than normal (e), leading to the induction of monomorphic sustained VT (f).
This case shows how, in the appropriate setting, a normally functioning pacemaker, with modern algorithms designed to avoid adverse events, can also be proarrhythmic.
Conflicts of interestThe authors have no conflicts of interest to declare.