Venous thromboembolism (VTE) is a relatively common complication during hospital stay and determination of VTE risk is critical to choosing the best prophylactic strategy for each patient.
ObjectivesIn the present study we studied the risk profile for VTE in hospitalized patients in a group of hospitals in Portugal.
MethodsBased on an open cohort of 4248 patients hospitalized in surgical, internal medicine, orthopedic or oncology departments, we determined thromboembolic risk at admission by applying a new score, modified from the Caprini and Khorana scores. Thrombotic, embolic and bleeding events and death were assessed during hospital stay and at three and six months after discharge.
ResultsThe median duration of hospital stay was five days and thromboembolic prophylaxis was implemented in 67.2% (n=2747) of the patients. A low molecular weight heparin was used as prophylaxis in the majority of cases (88.3%). Most patients were classified as high (68%) or intermediate risk (27%). The overall incidence of thromboembolic events was 1.5%. Major bleeding events were recorded in 3.89% of patients and all-cause mortality was 3.4%.
ConclusionsIn this study, we propose a modified VTE risk score that effectively risk-stratifies a mixed inpatient population during hospital stay. The use of this score may result in improvement of thromboprophylaxis practices in hospitals.
O tromboembolismo venoso (TEV) é uma complicação relativamente frequente, complica o internamento hospitalar, e a determinação do risco da ocorrência de TEV é essencial, de molde a permitir a escolha da melhor estratégia de profilaxia para cada doente.
ObjetivoEstudar o perfil de risco para TEV em doentes hospitalizados, num grupo de hospitais de Portugal.
MétodosNeste estudo, com a inclusão duma coorte aberta de 4248 doentes internados nos departamentos de cirurgia, medicina interna, ortopedia ou oncologia, foi determinado na admissão o risco tromboembólico de cada doente, através dum novo score, modificado dos scores de Caprini e de Khorana. Os eventos trombóticos, embólicos e hemorrágicos e morte foram registados durante o internamento e aos três e seis meses após a alta.
ResultadosA mediana do tempo de internamento foi de cinco dias e foi administrada terapêutica de profilaxia tromboembólica em 67,2% (n=2747) dos doentes, foi usada uma heparina de baixo peso molecular em 59,3% dos casos. A maior parte dos doentes foi classificada com um risco elevado (68%) ou intermédio (27%). A incidência global de eventos tromboembólicos foi de 1,5%. Foram registados eventos hemorrágicos maiores em 3,89% dos doentes e a mortalidade por todas as causas foi de 3,4%.
ConclusõesNeste estudo, os autores propõem um novo score para avaliação do risco de TEV que estratificou de modo efetivo uma população mista de doentes durante o internamento hospitalar. O uso desse score poderá resultar numa melhoria da prática da tromboprofilaxia nos nossos hospitais.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cardiovascular disease, affecting 1-2 per 1000 adults annually.1 The disease's consequences are significant and can be incapacitating, including morbidity, mortality, disability, and diminished health-related quality of life among affected individuals,1–3 as well as imposing a substantial socioeconomic burden on healthcare systems.4 Mortality in patients with untreated or undiagnosed PE has been reported to be as high as 30%,5 and it is the third most common cause of hospital-related death.6,7
While vascular endothelial damage, blood flow stasis, and hypercoagulability have been described as the three main components in the pathogenesis of venous thrombosis, advances in research have also identified many other transient and persistent risk factors – demographic (e.g. advanced age), biological (e.g. increased prothrombin and fibrinogen levels), behavioral (e.g. smoking), environmental (e.g. long-distance travel), and health condition-related (e.g. surgical and comorbid conditions, such as hypertension, diabetes or high cholesterol levels) – that interact simultaneously or sequentially in the development of VTE.8–10
VTE is of special concern in hospitalized patients.11–13 According to the current American College of Chest Physicians (ACCP)14 and European Society of Cardiology15 guidelines, it requires acute treatment with low molecular weight heparin (LMWH) or novel oral anticoagulants (NOACs) followed by at least three months of therapy with oral anticoagulants such as vitamin K antagonists or NOACs. This is a fairly effective treatment strategy for preventing VTE recurrence.
Assessment of VTE risk is essential to accurately identify patients who might benefit from VTE prophylaxis and to develop prevention strategies for optimal care. Although effective VTE prophylaxis can halve the risk of VTE,16 it is still largely underused in medical and surgical patients.17 In a population-based study,18 of all cases with venous thromboembolism, 24% had been previously admitted to a surgical ward and 22% to a medical ward.
Several clinical studies have shown the need to put into practice effective hospital strategies based on systematic and individualized assessment of VTE, and also to optimize the institution of proper prophylaxis in the context of in-hospital and outpatient management.
Information on VTE risk and prophylaxis practices in Portugal is scarce. Hence, the primary aim of this study was to assess the incidence of VTE risk in the hospital care setting in Portugal using a thromboembolic score based on a risk assessment model (RAM), adapted from Caprini et al.19 and Khorana et al.,20 whenever applicable (hereafter the ARTE-RAM score) (Table 1).
| Risk factor | Points |
|---|---|
| Age 40-60 years | 1 |
| Obesity (BMI >30 kg/m2) | 1 |
| Major surgery (<1 month) | 1 |
| Varicose veins | 1 |
| Smoking | 1 |
| CHF (present or <1 month) | 1 |
| Sepsis or severe infection (<1 month) | 1 |
| Acute pulmonary disease | 1 |
| COPD | 1 |
| Inflammatory bowel disease | 1 |
| Oral contraception or HRT | 1 |
| Pregnancy or postpartum (<1 month) | 1 |
| History of unexplained stillborn infant, miscarriage, premature birth with toxemia or growth-restricted infant | 1 |
| Minor surgery | 1 |
| Lung tumor | 1 |
| Lymphoma | 1 |
| Gynecologic cancer | 1 |
| Genitourinary or prostate cancer | 1 |
| Platelet count before chemotherapy ≤350000/μl | 1 |
| Hemoglobin <10 g/dl or RhEPO use | 1 |
| Leukocyte count before chemotherapy >11000/mm3 | 1 |
| Medical patient currently at bed rest | 1 |
| Age 61-74 years | 2 |
| BMI >35 kg/m2 | 2 |
| Cancer (active or previous) | 2 |
| Patient confined to bed (>72 h) | 2 |
| Immobilization | 2 |
| Central venous access | 2 |
| Major surgery (>45 min) | 2 |
| Laparoscopic surgery | 2 |
| Arthroscopic surgery | 2 |
| Gastric tumor | 2 |
| Pancreatic tumor | 2 |
| Age >74 years | 3 |
| History of DVT | 3 |
| Thrombophilia (per type) | 3 |
| Family history of DVT | 3 |
| Stroke (<1 month) | 5 |
| Major lower limb arthroplasty | 5 |
| Pelvic, hip or leg fracture (<1 month) | 5 |
| Acute spinal cord injury (paralysis) (<1 month) | 5 |
| Multiple trauma (<1 month) | 5 |
BMI: body mass index; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; DVT: deep vein thrombosis; HRT: hormone replacement therapy; RhEPO: recombinant human erythropoietin.
Secondary objectives were to characterize the profile of patients at risk for VTE, to determine the proportion of at-risk patients who receive effective prophylaxis (during hospitalization and after discharge), and to determine the incidence of thromboembolic and bleeding events (during hospitalization and after discharge).
MethodsThe detailed methodology of the ARTE (Avaliação de Risco de Tromboembolismo Venoso – Assessment of Venous Thromboembolism Risk) study has been previously published.21 Briefly, ARTE was a non-interventional, multicenter, prospective study performed from December 2008 to June 2012 at 14 Portuguese hospitals (listed in the Acknowledgements section). Inclusion criteria were patients aged ≥18 years hospitalized in one of the eligible medical departments (internal medicine, oncology, surgical or orthopedic), who signed informed consent to participate. The study excluded patients admitted with a diagnosis of VTE, or who were receiving chronic antithrombotic therapy.
Patients were enrolled sequentially in each participating center until the desired sample size of 4000 patients was achieved, and treated at the physician's discretion according to local clinical practice. Data were collected from hospital charts and the relevant information was entered onto standardized case report forms. Patient demographics, admission discharge diagnoses, risk factors for VTE (as defined in the ACCP guidelines22), risk factors for bleeding, duration of hospitalization, type of VTE prophylaxis and concomitant medication were obtained directly from patients at the time of enrollment and of hospital discharge. At the time of enrollment, the ARTE-RAM thromboembolic score was also calculated for each patient.
In order to assess the occurrence of thromboembolic and bleeding events, as well as cases of death or hospital readmission, patients were further contacted by telephone at six months after discharge.
Statistical analysisAn estimated 1000 patients were included per analysis subgroup to assess the true occurrence of VTE risk at a 95% confidence interval with a margin of error of 3%. Quantitative data were expressed as medians or means (and standard deviation) when appropriate, while categorical data were expressed as number and percentage of the population. Exploratory analysis was performed using chi-square testing for categorical variables and two-sided t tests for continuous variables. A p-value of less than 0.05 was considered statistically significant.
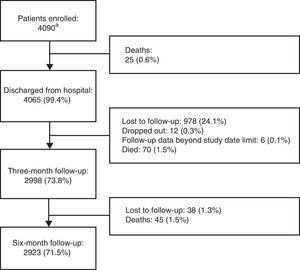
ResultsThe number of patients included and the study flow diagram are depicted in Figure 1.
Study flow diagram. aOf the total 4248 patients originally included, 158 were excluded, of whom 63 (39.9%) did not fulfill eligibility criteria, 44 (27.9%) had seriously incomplete records, 42 (26.6%) had no available weight data, and nine (5.7%) had no available hospital discharge and/or follow-up data.
A total of 4090 patients were enrolled (54% female), mean age 57 years, mean weight 71 kg, mean height 164 cm, mean body mass index 26.2 kg/m2, and 17% obese. Patients’ demographic and clinical characteristics are shown in Table 2.
Baseline demographic and clinical characteristics of the 4090 patients included.
| Demographic | |
| Female, % (n) | 54 (2209) |
| Age in years, mean (SD) | 57 (17) |
| Weight in kg, mean (SD) | 71 (15) |
| BMI in kg/m2, mean (SD) | 26.2 (4.7) |
| Body mass index >30 kg/m2, % (n) | 17 (695) |
| General aspects | |
| Family history of DVT, % (n) | 14.5 (592) |
| Patient confined to bed (>72 h), % (n) | 16.6 (690) |
| Immobilization, % (n) | 8.9 (364) |
| Central venous access, % (n) | 7.7 (314) |
| Previous history | |
| Stroke (<1 month), % (n) | 0.9 (36) |
| Deep vein thrombosis history, % (n) | 3.5 (144) |
| Cancer (active or previous), % (n) | 34.6 (1,416) |
| Major surgery (<1 month), % (n) | 3.8 (156) |
| Varicose veins, % (n) | 30.5 (1,248) |
| Smoking habits, % (n) | 19.7 (806) |
| Thrombophilia (per type), % (n) | 0.3 (10) |
| Clinical history | |
| CHF (present or <1 month), % (n) | 6.1 (248) |
| Sepsis or severe infection (<1 month), % (n) | 3.7 (152) |
| Acute pulmonary disease, % (n) | 6.5 (266) |
| COPD, % (n) | 4.1 (168) |
| Medical patient currently at bed rest, % (n) | 7.6 (311) |
| Inflammatory bowel disease, % (n) | 1.2 (48) |
| Gynecological/obstetric history | |
| Oral contraception or hormone replacement therapy, % (n) | 15.7 (343) |
| Pregnancy or postpartum (<1 month), % (n) | 1.1 (25) |
| History of unexplained stillborn infant, miscarriage, premature birth with toxemia or growth-restricted infant, % (n) | 4.6 (100) |
| Surgical patients | |
| Major surgery (>45 min), % (n) | 47.3 (1,935) |
| Laparoscopic surgery, % (n) | 8.2 (336) |
| Arthroscopic surgery, % (n) | 3.9 (161) |
| Minor elective surgery, % (n) | 9.9 (403) |
| Orthopedic patients | |
| Major lower limb arthroplasty, % (n) | 7.2 (294) |
| Pelvic, hip or leg fracture (<1 month), % (n) | 3.3 (134) |
| Acute spinal cord injury (paralysis) (<1 month), % (n) | 0.4 (18) |
| Multiple trauma (<1 month), % (n) | 0.5 (20) |
| Cancer patients under chemotherapy | |
| Gastric tumor, % (n) | 9.0 (130) |
| Pancreatic tumor, % (n) | 1.4 (20) |
| Lung tumor, % (n) | 3.9 (57) |
| Lymphoma, % (n) | 1.4 (20) |
| Gynecologic cancer, % (n) | 16.2 (129) |
| Genitourinary or prostate cancer, % (n) | 10.2 (148) |
| Platelet count before chemotherapy ≤350000/μl, % (n) | 4.9 (71) |
| Hemoglobin <10 g/dl or RhEPO use, % (n) | 6.0 (87) |
| Leukocyte count before chemotherapy >11000/mm3, % (n) | 3.7 (54) |
BMI: body mass index; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; DVT: deep vein thrombosis; HRT: hormone replacement therapy; RhEPO: recombinant human erythropoietin.
The proportions of patients included in each of the four medical areas considered were 38.4% (n=1570) in oncology, 24.6% (n=1008) in general surgery, 18.7% (n=764) in internal medicine and 18.3% (n=748) in orthopedics. Of the total population, 592 patients (14.5%) had a family history of thromboembolism. The mean length of hospital stay was 8.3±18.1 days (median 5), and the major reasons for hospitalization were cancer (15.0%), gastrointestinal/hepatobiliary disease (8.5%) and acute non-infectious respiratory disease (6.1%). Six-month follow-up was obtained in 2923 (71.5%) patients.
The incidence of the ARTE-RAM scores obtained at admission in the overall population is shown in Table 3.
According to the predefined classes of thromboembolic risk, 192 patients (4.7%) were considered to have low risk for VTE (ARTE-RAM score of 0 or 1), 1090 (26.7%) intermediate risk (ARTE-RAM score of 2, 3 or 4) and 2808 (68.6%) had an ARTE-RAM score ≥5, and were considered at high risk for VTE.
Considering the different medical areas individually, the distribution of the risk classes was as shown in Table 4. A high risk of VTE (ARTE-RAM score ≥5) was more prevalent among oncology patients (88.2%), followed by orthopedic patients (72.5%), surgical patients (54.6%) and internal medicine patients (43.2%).
Distribution of risk classes by medical area.
| Risk classes (ARTE-RAM score) | Incidence n (%) (95% CI) | |||
|---|---|---|---|---|
| Internal medicine | Oncology | Orthopedic | Surgical | |
| Low risk (0-1) | 115 (15.1) (12.6-17.6) | 13 (0.8) (0.4-1.2) | 23 (3.1) (1.9-4.3) | 41 (4.1) (2.9-5.3) |
| Intermediate risk (2-4) | 319 (41.8) (38.3-45.3) | 172 (11.7) (10.1-13.3) | 182 (24.3) (21.2-27.4) | 417 (41.4) (38.4-44.4) |
| High risk (≥5) | 330 (43.2) (39.7-46.7) | 1385 (88.2) (86.6-89.7) | 543 (72.5) (69.3-75.7) | 550 (54.6) (51.5-57.6) |
CI: confidence interval.
Overall, 2747 patients (67.2%) received VTE prophylaxis during hospitalization, and 592 (20.3%) of those with follow-up received VTE prophylaxis after discharge. During hospitalization, VTE prophylaxis was most frequently prescribed for orthopedic and oncology patients (76.5% and 72.0%, respectively), and LMWH was the most commonly used type of prophylaxis (88.3% of the 2747 treated inpatients), for a mean of 7.8±10.6 days (median 6). In 12.3% (n=505) of the patients, LMWH was maintained after discharge from hospital. Regarding the outpatient setting, among the 2923 patients with six-month follow-up, VTE prophylaxis was also most frequently recorded in orthopedic and oncology patients (36.2% and 14.3%, respectively), and LMWH was also the most commonly prescribed type of prophylaxis (85.3% of the 592 treated outpatients) (Table 5).
Thromboembolic prophylaxis.
| Inpatients (n=4090) | Outpatients with follow-up (n=2923) | |||
|---|---|---|---|---|
| No. of patients | (%) | No. of patients | (%) | |
| Treated patients | 2747 | 67.2 | 592 | 20.3 |
| Unfractionated heparin | 18 | 0.4 | 2 | 0.1 |
| LMWH | 2425 | 59.3 | 505 | 17.2 |
| Xa inhibitors | 1 | - | 32 | 1.1 |
| VKA | 20 | 0.5 | 34 | 1.2 |
| Other measures (elastic compression stockings) | 622 | 15.2 | 41 | 1.4 |
LMWH: low molecular weight heparin; VKA: vitamin K antagonists.
In the study population, 59 patients (1.4%) had contraindications for pharmacological prophylaxis, 26 (44.1%) of whom were hospitalized in internal medicine departments. Even though contraindication for anticoagulation was identified, 22 patients received prophylaxis during hospitalization, and three during follow-up.
The overall incidence of thromboembolic events was 1.5%, corresponding to 60 patients, 22 (0.58%) during hospital stay and 36 (0.93%) during the six-month follow-up period. The most common types of VTE during hospitalization were deep vein thrombosis (n=10), ischemic stroke (n=6) and pulmonary embolism (n=5), and deep vein thrombosis (n=13) and ischemic stroke (n=13) during follow-up. Overall, the majority of VTE occurred in oncology and internal medicine patients (n=29 and n=21, respectively). Analysis of the occurrence of VTE during hospitalization and follow-up periods separately reveals a significantly greater number of VTE episodes in internal medicine patients during hospitalization (n=13, p<0.001) and also more in oncology patients during follow-up (n=21), although this was not statistically significant (p=0.055).
A total of 327 patients (8%) had bleeding events, in 216 of whom the event occurred during hospitalization, 106 during follow-up, and five during both periods. Major bleeding events were observed in 159 (3.9%) of hospitalized patients, and in 68 (2.3%) of outpatients. The most frequent major bleeding events during hospitalization were ≥2 g/dl hemoglobin decrease (n=142), blood transfusion (n=98), intestinal and gastric bleeding (n=17 and n=12, respectively) and intestinal bleeding (n=63), ≥2 g/dl hemoglobin decrease (n=7) and blood transfusion (n=6) during follow-up.
Mortality from any cause over the six-month study period was 3.4%.
DiscussionThe validation of a RAM modified from the Caprini and Khorana scores in a heterogeneous hospitalized population that includes medical and cancer patients may be useful in clinical practice. Given the complexity of a significant number of medical and cancer patients, an ideal model must accurately identify a threshold for the risk of VTE and predict the correct risk level in heterogeneous patients at admission, irrespective of their initial or final diagnosis.23 One attempt to develop such a risk score in patients with active cancer in chemotherapy was the Protecht score.24 This score requires a P-selectin assay, which may limit its practical use,25 and is only included here for reasons of comparison. The ARTE-RAM score presented in this study was based on both well-established and novel risk factors, aiming for a consensual version of a tool that can be widely used in daily clinical practice, responding to a perceived need in the medical community.26
The ARTE study, including populations admitted to general surgery and medicine departments, was designed to assess a new risk scoring method and to demonstrate the value of individual risk assessment for a broad range of patients. The study was conducted in a cohort of patients hospitalized in four departments (internal medicine, oncology, surgical or orthopedic) of 14 hospitals in Portugal.
Overall, the study demonstrated a high prevalence of high risk for VTE (68% of all patients), which is considerably higher than the overall risk for VTE (52.7%) found in the Portuguese patients included in the ENDORSE study.27 This difference may be due to the especially high prevalence of VTE risk among oncology and orthopedic patients found in our study.
The 67.2% of all in-hospital patients who received some kind of thromboprophylaxis in the ARTE study is an improvement over the 58.5% treated patients found in the Portuguese ENDORSE subgroup, which may reflect an increase in awareness on this subject.
Despite the variety of prophylaxis measures, 45 of the 2747 treated patients (1.6%) suffered VTE. In a paper by Zakai et al.,28 the incidence of VTE was 7.6 per 1000 admissions. If ischemic stroke is excluded, we found an incidence of 3.9 VTE events per 1000 admissions. This difference could be explained by a higher percentage of prophylaxis in our study (67.2% vs. 52% in Zakai et al).
The ARTE study corroborates other reports29 that alert the medical community to the need for extended VTE prophylaxis beyond hospital discharge. In fact, the majority of VTE events were recorded in the six-month follow-up period (0.93% of all patients).
Major bleeding events were mainly observed during hospital stay, when close medical vigilance enables immediate intervention to minimize its consequences. Of inpatients receiving anticoagulant prophylaxis, only 3.89% had a major bleeding complication. This underlines the need for accurate risk estimation before the introduction of pharmacological VTE prophylaxis, in order to offer an appropriate strategy for each patient and especially to avoid underuse of anticoagulants based on wrongly perceived clinical assumptions of risk for bleeding complications.
Several studies, including a Cochrane review, have evaluated the importance of interventions designed to increase the implementation of thromboprophylaxis in hospitalized adult medical and surgical patients. The Cochrane review, published in 2013,30 analyzed 55 studies, including randomized controlled trials and observational studies, that implemented a variety of system-wide strategies aimed at improving thromboprophylaxis rates in many settings and patient populations. The authors found statistically significant improvements in prescription of prophylaxis therapies associated with education, alerts and multifaceted interventions. Multifaceted interventions with an alert component seems to be the most effective. Another review analyzed ambulatory cancer patients receiving chemotherapy31 and confirmed the importance of primary thromboprophylaxis in reducing the incidence of symptomatic VTE.
Worldwide, the search for cost-effective strategies for prevention of VTE has been a fundamental concern in recent years. There is, however, no agreement as to the best approach on this issue. On one hand there is solid evidence showing the effectiveness of computerized decision support for the implementation of thromboprophylaxis. On the other hand, there have been doubts about its cost-effectiveness.
A study published in 201132 assessed a four-year period in a Spanish hospital and demonstrated that the implementation of e-alerts led to a net cost saving of €6.50 per hospitalized patient and concludes that if all hospitalized patients in Spain were considered, the total yearly savings would approach €30 million.
LimitationsThis study has several limitations. Firstly, although we made an initial assessment of risk factors at time of admission, identified by qualified physicians, there may have been intersite variability in data collection. Secondly, for the purpose of classifying patients in disease categories, we used the admission diagnosis as the main diagnosis, accepting that, in some cases, this diagnosis could be wrong or the clinical course of the disease episode could modify the relative effect of VTE risk factors (for example, a patient could be admitted for an acute condition attributed to cancer but undergo surgical intervention during hospital stay). Thirdly, clinical diagnosis of DVT and PE is known to be unreliable and clinical examination alone underestimates the true incidence of VTE,33 particularly in an outpatient setting, which may have contributed to underestimation of asymptomatic VTE events and hence of the true incidence of VTE. Finally, a large number of patients were lost to follow-up, hampering analysis of thromboprophylaxis and clinical outcomes after hospital discharge.
ConclusionsThe ARTE study demonstrated that a large proportion of hospitalized patients are at high risk of VTE. Thromboprophylaxis was administered to a significant number of these patients but its use may be less desirable in the outpatient setting. Validation of the ARTE-RAM score in future studies may support the ARTE study's findings and lead to its use when combined with appropriate information technology.
DisclosuresThe ARTE study was sponsored by Sanofi and promoted by ForPoint - Instituto de Formação e Inovação na Saúde. Editorial assistance was provided by KeyPoint - Consultoria Científica.
Funding sourcesThe ARTE study was granted an unconditional financial grant by Sanofi Portugal.
Conflicts of interestD. Ferreira, J. A. De Sousa, P. Felicissimo, and A. França formed the scientific committee of the ARTE study and have received honoraria from Forpoint – Institute for Training and Innovation in Health.
The authors declare that they all contributed to the concept and design of the study and to the interpretation of data, as well as to the writing, revising and final approval of the version to be published.
The authors acknowledge the principal investigators who participated in the study: Dr. Aida Paulino (Hospital Amato Lusitano); Dr. Belarmino Clemente (Hospital Santa Cruz); Dr. Daniel Ferreira (Hospital da Luz); Dr. Dialina Brilhante (Instituto Português de Oncologia de Lisboa – Francisco Gentil); Dr. Diogo Silva Gomes (Hospital de Faro); Dr. João Pacheco Pereira (Hospital Egas Moniz); Dr. Joaquim Abreu de Sousa (Instituto Português de Oncologia do Porto – Francisco Gentil); Dr. Joaquim Lebre (Centro Hospitalar Vila Nova de Gaia/Espinho); Dr. Jorge Roldão Vieira (Hospital Garcia de Orta); Dr. Lúcia Borges (Hospital Infante D. Pedro); Dr. Luís Branco Amaral (Hospital Curry Cabral); Dr. Paulo Felicíssimo (Hospital Prof. Doutor Fernando Fonseca); Dr. Pedro Carvalho (Hospital São Francisco Xavier); Dr. Rubina Silva (Hospital Pedro Hispano).