To determine whether right ventricular and/or atrial speckle tracking strain is associated with previous arrhythmic events in patients with repaired tetralogy of Fallot.
Methods and ResultsWe studied right ventricular and atrial strain in 100 consecutive patients with repaired tetralogy of Fallot referred for routine echocardiographic evaluation. Patients were divided into two groups, one with previous documentation of arrhythmias (n=26) and one without arrhythmias, in a median follow-up of 22 years. Patients with arrhythmias were older (p<0.001) and had surgical repair at an older age (p=0.001). They also had significantly reduced right ventricular strain (-14.7±5.5 vs. -16.9±4.0%, p=0.029) and right atrial strain (19.1±7.7% vs. 25.8±11.4%, p=0.001). Neither right ventricular nor right atrial strain were independent predictors of the presence of a history of documented arrhythmias, which was associated with age at correction and with the presence of residual defects. In a subanalysis after excluding 23 patients who had had more than one corrective surgery, right ventricular strain was an independent predictor of the presence of previous arrhythmic events (OR 1.19, 95% CI 1.02-1.38, p=0.025). Right atrial strain was also an independent predictor after adjustment (OR 0.93, 95% CI 0.87-0.99, p=0.029). The ideal cut-off for right ventricular strain was -15.3% and for right atrial strain 23.0%.
ConclusionsCompared with conventional echocardiographic parameters, strain measures of the right heart are associated with the presence of arrhythmic events, and may be useful for risk stratification of patients with repaired tetralogy of Fallot, although a prospective study is required.
Avaliar se o strain por speckle tracking da aurícula e/ou ventrículo direito se associa com eventos arrítmicos prévios em doentes com tetralogia de Fallot reparada.
Métodos e resultadosAnalisámos o strain auricular e ventricular direito em 100 doentes consecutivos com tetralogia de Fallot reparada em ecocardiograma de rotina. Dividiram-se os doentes num grupo com documentação prévia de arritmias (n=26) e outro sem arritmias, num seguimento mediano de 22 anos após cirurgia. Os doentes com arritmias tinham mais idade (p<0,001) e cirurgia reparadora numa idade maior (p=0,001). Apresentam também um strain ventricular direito significativamente reduzido (-14,7±5,5 versus -16,9±4,0%, p=0,029) tal como o strain auricular direito (19,1±7,7% versus 25,8±11,4%, p=0,001). Contudo, nem o strain auricular, nem o ventricular foram preditores independentes da presença de história prévia de arritmias documentadas. Estas associaram-se à idade da realização da correção e com a presença de defeitos residuais. Numa sub-análise após exclusão de 23 doentes que tiveram mais do que uma cirurgia corretiva, o strain ventricular direito foi preditor independente da presença prévia de arritmias (OR 1,19, IC 95% 1,02-1,38, p=0,025). O strain auricular direito é também preditor independente após ajustamento (OR 0,93, IC 95% 0,87-0,99, p=0,029). O limiar ideal para o strain ventricular direito é de – 15,3% e para o strain auricular direito de 23,0%.
ConclusõesComparativamente com os parâmetros ecocardiográficos convencionais, as avaliações do strain do coração direito associam-se à presença de eventos arrítmicos previamente documentados e poderá ser útil na estratificação de risco em doentes com tetralogia de Fallot reparada, embora seja necessário um estudo prospetivo.
First described in 1671 by Niels Stensen, tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect.1,2 Before the development of surgical palliation and correction, about 50% of patients with TOF died in the first few years of life and it was unusual for any to survive for more than 30 years.3 Early surgical repair enabled a dramatic improvement in the outcome of TOF, with most patients reaching adulthood.1,4,5
Previously, some patients received palliative surgery by construction of a systemic-to-pulmonary arterial shunt, balloon dilation, or placement of a stent in the right ventricular (RV) outflow tract in infants, deferring repair for later in life.1 A staged approach was then suggested, but this has potential disadvantages such as long-lasting right ventricular pressure and/or volume overload and persistent cyanosis. However, even after early surgical repair, complications may occur, mainly in adulthood, such as significant pulmonary valve regurgitation, aortic root dilation and arrhythmias.1 Arrhythmias are in fact a considerable clinical problem in adults with repaired TOF, with recent studies showing incidences of sustained tachyarrhythmias as high as 43%.6
Ventricular and atrial dilation and dysfunction are associated with arrhythmias. The gold standard for right heart evaluation is cardiac magnetic resonance,1,2 because it enables better global and regional functional analysis of the right heart. However, it is often not available in clinical practice, and echocardiography is still the most commonly used technique. Two-dimensional speckle tracking echocardiography is an early marker of ventricular dysfunction and can thus be useful in the routine evaluation of TOF patients.8–11
Left ventricular (LV) longitudinal function by two-dimensional speckle tracking echocardiography is a predictor of life-threatening ventricular arrhythmias and death in patients with heart disease, including adults with repaired TOF.8,9 Since the disease mainly involves the right heart, we aimed to determine if RV and right atrial (RA) strain evaluated by two-dimensional speckle tracking echocardiography is associated with previously documented arrhythmic events (ventricular and supraventricular) in patients with repaired TOF.
MethodsAll patients with repaired TOF followed at the adult congenital heart disease outpatient clinic of Santa Marta Hospital (Lisbon, Portugal) who underwent routine transthoracic echocardiography between 2008 and 2015 with digitally stored images of sufficient quality were prospectively included in the study. Clinical characteristics were obtained by review of medical files and included age, gender, date and type of surgical procedures, electrocardiographic (ECG) data, and New York Heart Association functional class at the time of the echocardiogram. The primary endpoint was documentation of supraventricular or ventricular arrhythmias by 12-lead ECG, ambulatory ECG monitoring or other rhythm monitoring devices at any time in the follow-up after surgical correction. Single or paired premature beats were not considered as an endpoint.
EchocardiographyA complete standard echocardiographic study was performed including M-mode, two-dimensional and Doppler study. It also included a specific echocardiographic evaluation of the right heart, using commercially available systems (Vivid 7™ and Vivid 9™, General Electric Healthcare). The study was performed with the patient in left lateral position using a 3.5 MHz transducer. For RV study, fractional area change was calculated from apical 4-chamber view as the percentage change in RV end-diastolic and end-systolic area. RV end-diastolic diameter was assessed at the mid level of the RV cavity in 4-chamber view. RA volume and tricuspid diastolic and systolic flow were also evaluated in apical 4-chamber view. Tricuspid annular plane systolic excursion (TAPSE) was defined as the maximum systolic motion of the lateral tricuspid annulus along its longitudinal plane. RV myocardial performance was measured according to the Tei index.12 Tissue Doppler echocardiography was used to assess longitudinal myocardial velocities at the mitral and tricuspid annulus.
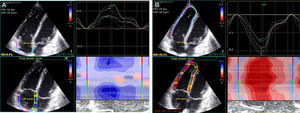
Images were acquired in apical 4-chamber view, and the transducer settings of the B-mode image were adjusted to achieve a frame rate of at least 55 frames per second (fps) (preferably 60-80 fps). The grayscale definition was changed as necessary to optimize two-dimensional endocardial and myocardial right heart definition. Images were digitally stored in cineloop format with three sequential beats and transferred to a workstation for subsequent offline analysis using a customized software package (EchoPAC™, General Electric Healthcare). With this software, the endocardial border of the right ventricle and right atrium were manually traced and the program automatically generated additional lines near the epicardium. Speckles were then tracked, frame by frame, during the cardiac cycle. The region of interest was also adjusted manually by the operator whenever necessary. Manual correction was performed in the event of insufficient or incorrectly tracked segments. The right ventricle and right atrium were automatically divided into six segments and mean peak longitudinal strain was averaged from all six segments of the free wall and septum (Figure 1). Peak systolic longitudinal strain was synchronized with the ECG corresponding with pulmonary valve closure. Strain measurements were made by a single operator blinded to the patient's arrhythmic status. In patients with atrial fibrillation or atrial flutter, RA strain was not analyzed.
Statistical analysisContinuous variables are presented as mean or median, standard deviation or interquartile range (25th-75th percentile), as required. Normality and homogeneity of variance were tested with the Kolmogorov-Smirnov test and Levene's test, respectively. Categorical variables are reported as percentages. Differences between groups for categorical variables were tested with the chi-square test or Fisher's exact test, as appropriate. The two-tailed Student's t test was used to compare continuous variables with normal distribution and the Mann-Whitney test to compare non-normally distributed variables. The association between continuous variables was analyzed with Person's correlation. Discriminative ability was assessed by the area under the receiver operating characteristic (ROC) curve. The best cut-off points of both parameters (RV and RA strain) for arrhythmic events were selected by identification of the minimum Euclidean distance in ROC curve analysis.
Multivariate logistic regression models were used to assess the association of RV and RA strain with arrhythmic events. Factors that remained significant at the 0.05 level in univariate analysis were considered to be significant contributors and were included in the final model. Multicollinearity was also assessed and when identified, those variables were removed from the final multivariate model. Estimates of the association between predictors and endpoints are presented as odds ratios (OR) with 95% confidence interval (CI). The model's predictive ability was assessed with the Hosmer-Lemeshow test.
To establish and quantify the reproducibility of RV and atrial strain, agreement and intra- and interobserver reproducibility were assessed using the interclass correlation coefficient and reliability coefficients (Cronbach's alpha) with two-way mixed effects models. Mean differences and limits of agreement were analyzed with Bland-Altman plots. Fifteen consecutive patients were selected for reproducibility analysis and the two operators were blinded for patient status and previous results.
IBM SPSS Statistics software, version 19.0.0.2 (SPSS Inc., Chicago, IL) and R statistical software, version 2.15.0, were used for all statistical analyses. All statistical tests were two-sided with a critical value of 0.05 for statistical significance.
ResultsThe study included 100 consecutive patients with repaired TOF. Another 12 patients were excluded due to inadequate images for off-line analysis. Mean age was 35±11 years and 66% were male. At a median follow-up of 22 years, there were 26 patients with documented arrhythmic events (16 with supraventricular arrhythmias, five with ventricular arrhythmias and five with both). Patients with arrhythmias were older, had surgical repair at an older age, more often had a second corrective surgery and were less often in sinus rhythm at assessment (Table 1). In the echocardiographic assessment, these patients more often had residual ventricular septal defect (VSD) and pulmonary valve prosthesis (all replaced in a second repair surgery), more severe tricuspid regurgitation, more enlarged right ventricle and right atrium and lower RV and atrial strain (Table 2).
Characteristics of the study population.
| Overall (n=100) | Arrhythmias (n=26) | No arrhythmias (n=74) | p | |
|---|---|---|---|---|
| Age (years) | 35±11 | 44±14 | 32±8 | <0.001 |
| Male (%) | 66.0 | 73.1 | 63.5 | 0.376 |
| Previous palliative surgery (%) | 29.0 | 23.1 | 31.1 | 0.439 |
| Age at corrective surgery (years) | 6 (4-12) | 12 (5-23) | 6 (3-9) | 0.001 |
| Second corrective surgery (%) | 23.0 | 38.5 | 17.6 | 0.029 |
| Sinus rhythm (%) | 95.0 | 80.8 | 100.0 | <0.001 |
| RBBB (%) | 99.0 | 100.0 | 98.6 | 0.551 |
| NYHA class (%) | 0.347 | |||
| I | 76.0 | 69.2 | 78.4 | |
| II | 24.0 | 30.8 | 21.6 | |
| Time correction-echo (years) | 22 (4-12) | 29 (20-34) | 24 (21-28) | 0.093 |
echo: echocardiographic assessment; NYHA: New York Heart Association; RBBB: right bundle branch block.
Echocardiographic data.
| Overall (n=100) | Arrhythmias (n=26) | No arrhythmias (n=74) | p | |
|---|---|---|---|---|
| Normal LV functiona (%) | 97.0 | 88.5 | 100.0 | 0.003 |
| Residual VSD (%) | 13.0 | 26.9 | 8.1 | 0.014 |
| Pulmonary valve prosthesis (%) | 25.0 | 50.0 | 16.2 | 0.001 |
| Pulmonary regurgitation ≥2 (%) | 49.0 | 46.2 | 50.0 | 0.419 |
| Tricuspid regurgitation ≥2 (%) | 16 | 34.6 | 9.5 | 0.026 |
| Mitral E/e’ | 8.9 (7.2-12.1) | 9.8 (7.8-14.9) | 8.3 (6.9-9.9) | 0.061 |
| RV diastolic area (cm2) | 29.1±8.3 | 30.3±6.8 | 28.6±8.8 | 0.375 |
| RV systolic area (cm2) | 18.2±5.9 | 19.7±4.5 | 17.7±6.3 | 0.145 |
| RV FAC (%) | 0.37±0.09 | 0.34±0.09 | 0.38±0.09 | 0.076 |
| RV dimension (mm) | 34.9±9.5 | 40.0±7.8 | 33.2±9.4 | 0.008 |
| RA volume/BSA (ml/m2) | 27.8 (22.2-42.9) | 41.6 (29.5-57.6) | 24.4 (20.9-32.4) | 0.007 |
| Tricuspid e’ (cm/s) | 9 (7-12) | 8 (6-11) | 10 (7-12) | 0.132 |
| Tricuspid s’ (cm/s) | 8 (8-10) | 7 (6-9) | 8 (7-18) | 0.051 |
| Tricuspid E (cm/s) | 81±25 | 73±11 | 85±29 | 0.051 |
| Tricuspid E/e’ | 9.1 (5.9-11.2) | 9.9 (6.4-11.8) | 8.7 (5.5-10.8) | 0.418 |
| Peak pulmonary gradient (mmHg) | 18 (12-26) | 22 (13-37) | 17 (12-24) | 0.097 |
| RV/RA gradient (mmHg) | 30 (24-37) | 34 (25-41) | 30 (24-35) | 0.141 |
| PASP (mmHg) | 19 (14-25) | 17 (13-24) | 19 (13-26) | 0.473 |
| TAPSE (mm) | 17.8±4.4 | 16.5±4.4 | 18.3±4.4 | 0.077 |
| Tei index | 0.22 (0.12-0.26) | 0.22 (0.10-0.27) | 0.22 (0.12-0.26) | 0.972 |
| RV strain (%) | −16.4±4.6 | −14.7±5.5 | −16.9±4.0 | 0.029 |
| RA strain (%)b | 24.1±10.9 | 19.1±7.7 | 25.8±11.4 | 0.001 |
BSA: body surface area; FAC: fractional area change; LV: left ventricular; PASP: pulmonary artery systolic pressure; RA: right atrial; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion; VSD: ventricular septal defect.
Reproducibility data for RV and atrial strain are presented in Table 3. Reproducibility was significantly better for RV strain than for RA strain. RV strain correlated with other RV function parameters such as RV fractional area change, TAPSE and tricuspid s’, as well as with RV end-diastolic dimension and RA strain (Table 4). RA strain also correlated with RV function parameters (tricuspid s’, TAPSE and Tei index) and RA volume. RV strain showed a reasonable predictive accuracy for the association with arrhythmias (area under the curve [AUC] 0.660, 95% CI 0.530-0.791), with a cut-off of -13.8% (sensitivity 54% and specificity 76%). RA strain had a similar accuracy (AUC 0.670, 95% CI 0.560-0.781), with a cut-off of 23.0% (sensitivity 58% and specificity 69%). In univariate analysis, RA strain (OR 0.94, 95% CI 0.89-0.98, p=0.009) and RV strain (OR 1.12, 95% CI 1.01-1.24, p=0.032) were both associated with arrhythmias. Other major predictors of arrhythmia occurrence were age at correction (OR 1.05, p=0.006), residual VSD (OR 4.17, p=0.020) and the presence of a pulmonary valve prosthesis (OR 5.17, p=0.001). None of the other RV function measures could predict the presence of arrhythmias: RV fractional area change (OR 0.013, p=0.080), TAPSE (OR 0.91, p=0.080), Tei index (OR 6.23, p=0.313) and tricuspid s’ (OR 0.83, p=0.075).
Reproducibility results for right ventricular and right atrial strain.
| ICC (consistency) | ICC (agreement) | Reliability coefficienta | Mean difference (absolute) | Limits of agreement (absolute) | |
|---|---|---|---|---|---|
| RV strain (intraobserver) | 0.98 (0.94-0.99) | 0.98 (0.93-0.99) | 0.989 | −0.42 | ±2.04 |
| RV strain (interobserver) | 0.93 (0.81-0.98) | 0.93 (0.82-0.98) | 0.966 | −0.35 | ±3.84 |
| RA strain (intraobserver) | 0.85 (0.62-0.95) | 0.86 (0.63-0.95) | 0.921 | −0.71 | ±9.14 |
| RA strain (interobserver) | 0.85 (0.62-0.95) | 0.83 (0.55-0.94) | 0.919 | 2.29 | ±9.48 |
ICC: interclass correlation coefficient; RA: right atrial; RV: right ventricular.
Correlations between continuous variables.
| RV strain (r) | p | RA strain (r) | p | |
|---|---|---|---|---|
| RV strain | - | - | −0.573 | <0.001 |
| RA strain | −0.573 | <0.001 | - | - |
| RV FAC | −0.432 | <0.001 | 0.321 | 0.001 |
| RV diastolic area/BSA | 0.139 | 0.169 | −0.143 | 0.155 |
| RV/BSA | 0.319 | 0.006 | −0.177 | 0.078 |
| Tricuspid e’ | −0.068 | 0.509 | 0.151 | 0.137 |
| Tricuspid s’ | −0.395 | <0.001 | 0.259 | 0.010 |
| RV/RA gradient | −0.001 | 0.991 | −0.042 | 0.679 |
| PASP | −0.154 | 0.129 | −0.018 | 0.858 |
| Peak pulmonary gradient | 0.100 | 0.320 | −0.008 | 0.937 |
| TAPSE | −0.317 | 0.001 | 0.258 | 0.010 |
| Tei index | 0.147 | 0.253 | −0.281 | 0.027 |
BSA: body surface area; FAC: fractional area change; LV: left ventricular; PASP: pulmonary artery systolic pressure; r: correlation coefficient; RA: right atrial; RV: right ventricular; TAPSE: tricuspid annular plane systolic excursion; VSD: ventricular septal defect.
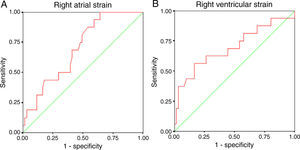
In the overall population and in multivariate regression analysis, neither RV nor RA strain were independent predictors of the presence of previous arrhythmias (Table 5). The main determinants were age at correction and the presence of a residual VSD and pulmonary valve prosthesis, which is a surrogate for subsequent right heart overload after the first repair. Since this right heart overload might bias our analysis and these patients had a second corrective surgery, we performed a subanalysis after excluding the 23 patients with a subsequent corrective surgery (18 with pulmonary valve prosthesis implantation and the remainder with closure of a significant residual VSD). The mean age of this subgroup was 35±10 years, and 16 patients had documentation of arrhythmias (supraventricular in 10 patients, ventricular in three and both in three). In this subgroup, after adjustment for patients’ age at repair and presence of a VSD, RV strain remained an independent predictor of the presence of arrhythmic events (OR 1.19, 95% CI 1.02-1.38, p=0.025) (Table 6). RA strain was also an independent predictor after adjustment (OR 0.93, 95% CI 0.87-0.99, p=0.029). ROC curve analysis showed similar predictive accuracy for both RV strain (AUC 0.696, 95% CI 0.532-0.860, cut-off -15.3%, sensitivity 62% and specificity 74%) and RA strain (AUC 0.699, 95% CI 0.571-0.827, cut-off 23.0%, sensitivity 59% and specificity 67%) (Figure 2). In all models, the predictive ability of the complete multivariate model was significantly higher, compared to each single variable.
Multivariate logistic regression analysis in the overall population.
| OR | 95% CI | p | |
|---|---|---|---|
| Model 1a | |||
| Age at correction | 1.05 | 1.01-1.09 | 0.014 |
| Residual VSD | 4.01 | 0.98-16.35 | 0.053 |
| Pulmonary prosthesis | 5.18 | 1.68-15.93 | 0.004 |
| RV strain | 1.08 | 0.96-1.22 | 0.187 |
| Model 2b | |||
| Age at correction | 1.05 | 1.01-1.09 | 0.022 |
| Residual VSD | 5.16 | 1.69-15.81 | 0.004 |
| Pulmonary prosthesis | 0.95 | 0.91-1.01 | 0.082 |
| RA strain | 0.95 | 0.91-1.01 | 0.082 |
CI: confidence interval; OR: odds ratio; RA: right atrial; RV: right ventricular; VSD: ventricular septal defect.
Predictive capacity (univariate) by AUC: age at correction (0.72), residual VSD (0.59), pulmonary prosthesis (0.67), RV strain (0.66), RA strain (0,67). Overall multivariate model predictive capacity: model 1 (0.80), model 2 (0.80).
Multivariate logistic regression analysis in the subset of patients without a second corrective surgery (n=77).
| OR | 95% CI | p | |
|---|---|---|---|
| Model 1a | |||
| Age at correction | 1.05 | 1.01-1.10 | 0.014 |
| Residual VSD | 1.97 | 0.35-11.09 | 0.441 |
| RV strain | 1.19 | 1.02-1.38 | 0.025 |
| Model 2b | |||
| Age at correction | 1.04 | 1.00-1.09 | 0.039 |
| Residual VSD | 2.03 | 0.36-11.61 | 0.424 |
| RA strain | 0.93 | 0.87-0.99 | 0.029 |
CI: confidence interval; OR: odds ratio; RV: right ventricular; RA: right atrial; VSD: ventricular septal defect.
Predictive capacity (univariate) by AUC: age at correction (0.72), residual VSD (0.57), RV strain (0.70), RA strain (0.70). Overall predictive capacity of multivariate model: model 1 (0.77), model 2 (0.77).
TOF is the most common form of cyanotic congenital heart defect (approximately 10% of all congenital heart defects – similar to a previous report by our center, which has a long experience in congenital heart disease).2,13 The prognosis of TOF has improved markedly in recent years due to advances in surgical techniques and early repair. In the 1990s, 32-year survival of patients who survived corrective surgery was 86%,4 whereas current long-term survival of repaired TOF is estimated to be >90% (30-year survival of 92%).5 There can however be important late complications following TOF repair including arrhythmias, heart failure and death.5 At 35-year follow-up, symptomatic arrhythmias have a cumulative incidence of 17%.5 Previous studies showed an incidence of supraventricular arrhythmias ranging between 4% and 20%, depending on the duration of follow-up.6,13–17 Repaired TOF is also associated with subsequent ventricular tachyarrhythmias, mortality and morbidity.15,18 The incidence of sudden death is 6% over 30 years, principally in the first few years after repair. The main predictors of arrhythmic events are older age at repair and residual RV structural abnormalities such as outflow aneurysms or significant pulmonary regurgitation and severe RV dilation with right or left ventricular dysfunction.9,14–16 Long-standing pulmonary regurgitation has deleterious effects on RV size and function, which increases the risk of severe arrhythmias and sudden death because RV function is a major prognostic factor in patients with operated TOF.9,14–16 After 35-year follow-up, 44% will require some type of reintervention, and at a median of 24 years after initial correction 40% will need pulmonary valve replacement.5
Due to the complicated geometry of the right ventricle, two-dimensional measurements of RV function may be inadequate and assessment is challenging. For this reason, cardiac magnetic resonance imaging (CMRI) is still the gold standard non-invasive technique for right heart structural and functional assessment in patients with congenital heart disease, including TOF.19 However, its limited availability, cost and contraindications are important limitations for its application in clinical practice, particularly for regular long-term follow-up of these patients, and echocardiography will remain the main tool for routine assessment of TOF patients.
Previous studies have validated TAPSE and tricuspid s’ measured by echocardiography against RV ejection fraction measured by CMRI, and both are currently used as measures of RV function.19 However, they did not take into account the multisegmental nature of contractility. Other parameters such as RV fractional area change in 4-chamber view, fractional shortening of the RV outflow tract and Tei index, although indicators of RV dysfunction, are less used.7 In a previous paper, our group showed the usefulness of new echocardiographic imaging techniques, particularly tissue Doppler imaging, in patients with repaired TOF and its association with arrhythmias.20 However, RV function was only assessed at the atrioventricular valve annular level. Recently it has also been demonstrated that LV longitudinal strain measured by two-dimensional speckle tracking echocardiography is an important indicator of mortality risk.8 This technique is not angle-dependent and is not affected by tethering effects. It can also be applied to RV functional assessment in patients with right heart disease, including patients with repaired TOF.21 It has been tested in patients with pulmonary hypertension, pulmonary embolism and atrial septal defects.22–24 However, very few studies have assessed RV function by two-dimensional speckle tracking echocardiography in adult patients late after TOF repair, and none has analyzed right atrial function. In our study, we performed a specific study of the right heart using conventional two-dimensional, Doppler and tissue Doppler methods, and also studied right atrial and ventricular function by peak longitudinal strain with two-dimensional speckle tracking echocardiography. This was measured in a single plane, by averaging six segments of the free wall and septum.
A pilot study performed at our center showed the potential value of these new technologies applied to the right heart to assess the association with arrhythmias in patients with repaired TOF.25 We therefore decided to enlarge the study sample to improve the statistical power of our conclusions. In the present study sample, 26% had a history of arrhythmias (ventricular in 10% and supraventricular in 21%), which is close to previous reports. These patients were older and had corrective surgery at an older age. They also more often underwent a second corrective surgery, mainly for pulmonary valve replacement. This confirms the important role of residual defects that lead to overload and hence enlargement and dysfunction of the right heart chambers after the first corrective surgery, which are known to cause arrhythmias. In our study population, RV systolic function as assessed by traditional methods, such as fractional area change, TAPSE, Tei index and tricuspid s’, were similar between groups. Although strain correlated with conventional RV function measures, we found significant reductions in both RA and RV strain in patients with arrhythmic events, confirming that this technique can detect contractile dysfunction earlier in the course of the disease.
RA and RV strain showed reasonable predictive ability, with an AUC >0.65. The cut-off obtained for RV strain was -13.8% with reasonable specificity and sensitivity. RA strain cut-off was 23.0%, also with reasonable sensitivity and specificity. Right heart chamber strain is a predictor for the presence of arrhythmias. However, after multivariate analysis with adjustment for other important predictors such as age at correction or the presence of residual VSD or pulmonary valve prosthesis (as a surrogate for previous significant pulmonary regurgitation), it was no longer a predictor. However, the predictive ability of the complete multivariate model was significantly higher, compared to each individual variable.
Although strain and strain rate improve significantly and acutely after correction of long-standing pulmonary regurgitation and residual stenosis, we do not know whether this improvement is maintained in long-term follow-up.26 We therefore excluded patients who underwent a second corrective surgery with pulmonary valve replacement (due to severe pulmonary regurgitation) and closure of a significant non-restrictive VSD. These previous defects are a surrogate marker for previous long-standing right heart overload that may have been the cause for the detected arrhythmias. We then performed a subanalysis of the 77 patients with only a single corrective surgery (20.8% with previous history of arrhythmia). In this group, 16.9% had a residual VSD, which was however restrictive in all cases. In this subanalysis, we confirmed the independent predictive value of RA and RV strain for the presence of previous arrhythmias.
LimitationsSince the arrhythmias were diagnosed before the echocardiographic assessment, this is considered a retrospective study. Thus, the present study cannot establish a definite causal relationship between strain and arrhythmias. In addition, the investigation and documentation of arrhythmias was symptom-driven, that is, only patients with symptoms suggestive of arrhythmias underwent subsequent investigation.
A prospective study on the association of right heart myocardial strain with future arrhythmic events would be more interesting. However, since most events occurred in late childhood or early adulthood, such a study would have to be performed in a pediatric cardiology setting. Also, the annual event rate is relatively low; this is a major difficulty in studies attempting to improve risk stratification in TOF patients, particularly with the use of recent technology, since it would require a large sample and long follow-up.
The number of patients with arrhythmias was relatively small, and it was not possible to study the types of arrhythmia separately due to low statistical power. Nonetheless, we performed a preliminary analysis in the substudy population, which showed that RV strain is the only predictor of ventricular arrhythmias (OR 1.36, 95% CI 1.07-1.73, p=0.011) and that RA strain is an independent predictor of supraventricular arrhythmias (OR 0.93, 95% CI 0.86-0.99, p=0.039) adjusted for age at correction and residual VSD (the other predictors in univariate analysis). None of the other measures of right ventricular or atrial function were predictors of either type of arrhythmia. This indicates that there is scope for further prospective studies.
Two-dimensional speckle tracking echocardiography strain was measured with non-dedicated software – we used an adaptation from LV two-dimensional speckle tracking echocardiography strain analysis. This could explain the results of our reproducibility analysis. Although the interclass correlation coefficient was excellent for RV strain and very good for RA strain (better for intraobserver variability), the limits of agreement by Bland-Altman analysis were rather wide. The use of non-dedicated software, with frequent use of manual corrections, may partially explain these results. The other possible explanation is related to image quality. Measurements were more reproducible when image quality was good. This suggests that follow-up assessments should be performed by the same operator and preferably with the same software, as recommended in a recent position paper from the European Association of Cardiovascular Imaging.27 In a study by Diller et al., TAPSE and RV two-dimensional systolic strain were not related to sudden cardiac death or life-threatening arrhythmia, in contrast to our results.9 One explanation is that that study only focused on ventricular arrhythmias, which in our study were found in only 10 patients (38%). Another possible explanation is the use of different software for strain quantification, since strain results are not interchangeable between different types of equipment.27
An analysis of strain rate might have been more appropriate, because it is less volume dependent. However, our speckle tracking analysis software did not enable regional strain rate analysis.
Recently described prognostic parameters, such as detailed two-dimensional assessment of the RV outflow tract or RV dyssynchrony by strain, were not analyzed, due to the retrospective nature of our study and the need for specific images.7,28 Also, we used LV strain analysis software, which is not yet validated for use in analysis of RV dyssynchrony.
ConclusionsIn patients with repaired TOF, age at correction and residual defects are significantly associated with previous documentation of arrhythmias. RA and RV strain are particularly useful in patients without significant residual defects after corrective surgery, because both are independently associated with the occurrence of arrhythmias. They seem to be an earlier marker of dysfunction than conventional measures. However, due to the retrospective nature of our study we could not confirm a direct causal relationship; further prospective long-term studies are needed, and this predictive value needs to be supported by more extensive prospective long-term data.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial supportThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.