A case of tricuspid valve infective endocarditis is presented. Since this was not the first episode, the patient had not undergone invasive procedures and there was no history of intravenous drug abuse, the possibility of congenital heart disease was considered, a hypothesis that was confirmed.
Os autores apresentam um caso de endocardite infecciosa (EI) recidivante da válvula tricúspide num doente sem hábitos toxicofílicos endovenosos. Esta entidade, embora mais prevalente nos doentes utilizadores de drogas endovenosas, pode ocorrer em indivíduos sem estes hábitos. A recorrência do processo infeccioso nesta localização, num doente sem aparentes fatores de risco, alertou para a possibilidade de haver uma cardiopatia congénita não conhecida, que se veio a confirmar.
Right-sided infective endocarditis mainly affects intravenous drug users,1 but it can be associated with a wide range of conditions such as alcoholism and immunodepression, use of permanent pacemakers or central venous catheters, and congenital heart disease.2,3 Its incidence ranges between 5% and 10%.4,5
Lejko-Zupanc et al. published a retrospective study in 1999 assessing all cases of infective endocarditis treated in the University Medical Center Ljubljana over a 12-year period. Of a total of 205 patients, 13 had right valve involvement, mainly the tricuspid valve. In no case was there a history of intravenous drug use. Four patients had a history of invasive procedures (abortion or placement of central venous catheter, intrauterine device or permanent pacemaker), and four suffered from a debilitating or malignant disease (alcoholic cirrhosis, essential thrombocytosis or laryngeal carcinoma). Two patients had previous valve disease, one had a ventricular septal defect (VSD), one had a history of endocarditis involving a pacemaker lead and another had no history of either heart disease or invasive procedures. Pulmonary embolism occurred in nine of the 13 patients, most of them without radiographic characteristics of pulmonary infarction but with infiltrates in the lungs. The case associated with VSD was a 33-year old woman, who subsequently underwent surgical closure of the defect and was cured.6
While intravenous drug use is considered the main risk factor for endocarditis of the right heart, in its absence congenital heart disease is the most likely cause of infectious valve disease.7–10
Case reportJCSF, a 49-year-old white male, married, employed as a medical auxiliary, was admitted to the emergency department with constricting chest pain at rest but no other symptoms.
He began suffering fatigue three months before this admission and had undergone laboratory tests and M-mode and two-dimensional echocardiography, which revealed no abnormalities. At that time, in view of a history of infective endocarditis at age 18, he was treated as an outpatient with intramuscular penicillin.
His fatigue worsened on minimal exertion and he lost 9 kg in weight in three months. In light of this continuing situation he consulted his family doctor and underwent further laboratory tests, which showed microcytic and hypochromic anemia, for which he was prescribed oral ferrous sulfate.
The patient went to the emergency department due to worsening symptoms, with an episode of constricting chest pain at rest, without radiation, of short duration and self-limited, but no other symptoms. He reported fatigue on minimal exertion, and one episode of syncope.
On physical examination in the emergency department the patient appeared thin and pale and a holosystolic murmur was audible over the mitral and tricuspid valves.
Laboratory tests showed microcytic hypochromic anemia, with red cell count 2.88 × 1012/l, hemoglobin (Hb) 7.5 g/dl, hematocrit 23.1%, with mean corpuscular volume (MCV) 79 fl, and renal failure with urea 96 mg/dl and creatinine 3.3 mg/dl, but no abnormalities in other laboratory parameters, including cardiac markers.
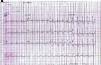
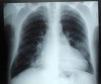
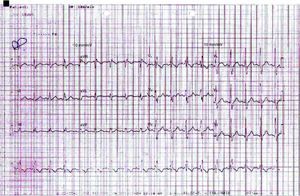
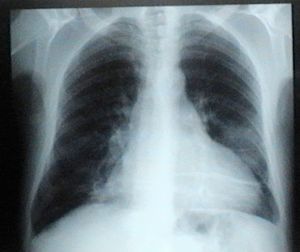
The electrocardiogram in the emergency department (Figure 1) showed sinus rhythm, heart rate of 106 bpm and complete right bundle branch block, and the chest X-ray (Figure 2) showed slight opacification of the lower half of the left hemithorax, a relevant finding given the clinical context.
He was admitted to the internal medicine department to clarify the clinical situation.
The patient was born in Lisbon, where he lived with his wife and child in a house with basic sanitation and good hygiene, and was working as a medical auxiliary at the time of admission.
He had had hepatitis A in childhood and an episode of tricuspid valve endocarditis at age 18, for which he was admitted to Hospital Pulido Valente. He was unable to specify what treatment he received at the time. He denied recurrent tonsillitis, and drug abuse, tobacco or alcohol consumption or promiscuous sexual habits (past or present). He had mild chronic renal failure of unknown cause of several years’ evolution. He had not undergone any dental procedures in the six months prior to this hospitalization.
Physical examination on the ward showed the patient to be underweight, dehydrated, pale with pale mucosa, but acyanotic, anicteric and apyretic. His body mass index was 18.5 kg/m2 (weight 54.8 kg, height 1.72 m). His blood pressure was 136/82 mmHg, with a regular pulse of 105 ppm. A rapid neurological examination showed no alterations. There was no jugular venous distension or carotid murmurs, and no visible cutaneous or sclerotic changes. He had halitosis but no evidence of dental caries or other oral lesions.
Cardiac auscultation revealed a grade IV/VI holosystolic murmur, more audible over the mitral and tricuspid valves and radiating over the entire precordial region, with no pericardial friction rub. Pulmonary auscultation showed normal breath sounds, with no adventitious sounds. The abdomen was soft, with no organomegaly or palpable masses. Pedal pulses were palpable and symmetrical; there was no lower limb edema or signs compatible with deep vein thrombosis, or other alterations.
Laboratory tests performed on the ward (Table 1) showed anemia compatible with chronic disease (Hb 6.5 g/dl, hematocrit 19.7%, MCV 78.5 fl, serum iron 41 μg/dl, total iron binding capacity 139 μg/dl, and ferritin 1008.1 ng/ml), mild thrombocytopenia and elevated inflammatory markers (C-reactive protein [CRP] 18.3 mg/dl and erythrocyte sedimentation rate [ESR] 123 mm); viral serology was negative, as was VDRL.
Laboratory tests performed on the ward.
| Red cell count 2 510 × 109/l, Hb 6.5 g/dl, hematocrit 19.7%, MCV 78.5 fl |
| White cell count 7 400 × 106/l, N 76%, platelets 149 000 × 106/l |
| CRP 18.3 mg/dl (N <0.5), ESR 123 mm |
| PTT 11.2/11 s, APTT 27.3/28 s, D-dimers 2.47 μg/ml (RV 0.0-0.5), fibrinogen 382 mg/dl (RV 200-400) |
| Serum iron 41 μg/dl (RV 65-175), TIBC 139 μg/dl (RV 250-425), ferritin 1008.1 ng/ml (RV 23.9-336) |
| Vitamin B12 778 pg/ml (RV 180-914), folate 3.5 ng/ml (RV 2.0-20.0) |
| Serology: Negative for CMV, EBV, HAV, HBV and HCV, VDRL, and HIV 1 and 2 |
APTT: activated partial thromboplastin time; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; MCV: mean corpuscular volume; PTT: prothrombin time; RV: reference value; TIBC: total iron binding capacity.
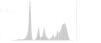
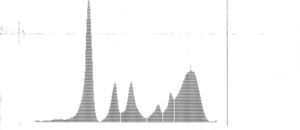
Other laboratory tests (Table 2) showed normal thyroid function, negative rheumatoid factor, and circulating immune complexes and complement analysis within references values. Serum protein electrophoresis (Figure 3 and Table 2) revealed hypoalbuminemia and an inflammatory pattern.
Other laboratory parameters.
| TSH 3.22 μU/ml (RV 0.35-5.5), FT3 2.35 pg/ml (RV 2.0-4.5), FT4 1.11 ng/dl (RV 0.89-1.8) |
| Rheumatoid factor: negative |
| Circulating immune complexes 4.3 μg/ml (<5.0), C3 1.06 g/l (RV 0.9-1.8), C4 0.2 g/l (RV 0.1-0.4), CH50 54.5 U/ml (RV 35-63) |
| Serum protein electrophoresis: albumin 22.5 g/l (RV 39-46); globulins: alpha 1 6.2 g/l (RV 2.5-5.0), alpha 2 9.3 g/l (RV 5.0-8.0), beta 1 3.2 g/l (RV 4.0-6.0), beta 2 4.8 g/l (RV 2.0-4.5), gamma 24.0 g/l (RV 7.5-13.5); albumin/globulin ratio 0.47 g/l (RV 1.15-2.18) |
RV: reference value; TSH: thyroid-stimulating hormone.
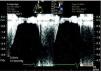
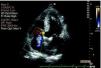
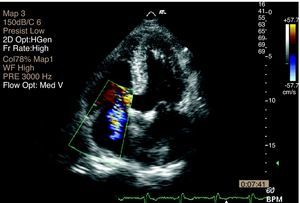
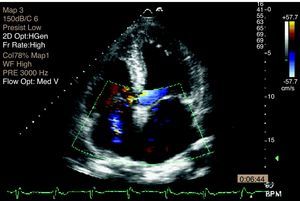
Transthoracic echocardiography (TTE) performed at the beginning of hospital stay showed two mobile structures adhering to the tricuspid valve leaflets, compatible with vegetations, to be clarified by transesophageal echocardiography (TEE). Moderate tricuspid regurgitation was documented, as well as pulmonary hypertension, with pulmonary artery systolic pressure (PASP) of 68 mmHg (Figures 4 and 5).
In the light of the above clinical, laboratory and echocardiographic findings, a diagnosis was made of subacute infective endocarditis and decompensated chronic renal failure.
Four aerobic blood cultures were performed and empirical therapy was begun with 3 000 000 U intravenous penicillin G sodium alternating with potassium every four hours for four weeks. In view of the patient's renal disease, a single 140-mg dose of gentamicin was administered. Penicillin- and gentamicin-sensitive Streptococcus sanguis was isolated in all blood cultures.
TEE confirmed the presence of vegetations affecting the tricuspid valve leaflets, with moderate regurgitation (Figure 6).
In view of the patient's microcytic hypochromic anemia, further diagnostic exams were performed to identify other possible causes, including abdominal ultrasound, upper gastrointestinal endoscopy (UGIE), colonoscopy and thoracic-abdominal computed tomography (CT).
Abdominal ultrasound showed no significant alterations, while UGIE revealed erosive antritis and bulbitis, which anatomopathological study showed to be compatible with non-active chronic gastritis, with no Helicobacter pylori bacilli. Colonoscopy identified a small rectal polyp, and anatomopathological study showed mild lymphocytic-plasmacytic infiltration and some evidence of focal epithelial hyperplasia, but no signs of malignancy. Thoracic-abdominal CT detected two triangular opacities in the peripheral parenchyma of the lung bases, suggestive of pulmonary infarctions associated with adjacent atelectasis.
There was marked improvement in the patient's clinical condition and laboratory parameters during hospitalization. He no longer complained of fatigue and regained his appetite on the second day of hospital stay. His general condition progressively improved; he had a single episode of fever (axillary temperature 38.4°C) on the third day, but thereafter was apyretic.
After a month of antibiotic therapy, there was clear improvement in his anemia, reduction in inflammatory markers, and reversal of thrombocytopenia, with the following laboratory results: Hb 10.4 g/dl, MCV 87.0 fl, mean corpuscular hemoglobin 29.3 pg, white cell count 8.17 × 109/l, neutrophils 66.8%, lymphocytes 23.4%, platelets 215 × 109/l, ESR 10 mm, CRP 3.45 mg/dl, urea 72 mg/dl, creatinine 2.0 mg/dl, and lactate dehydrogenase 348 U/l.
Two blood cultures performed after one month were negative, and resolution of the tricuspid valve infective lesions was observed.
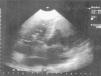
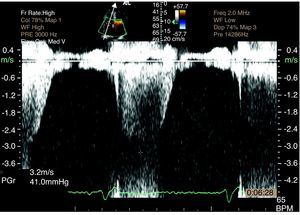
TTE reassessment three months later showed no valve vegetations and improvement in tricuspid regurgitation from moderate to mild, with PASP of 55 mmHg. High velocity (6 m/s) turbulent flow was still observed at the level of the upper interventricular septum (IVS), compatible with VSD (Figure 7). TEE confirmed a small restrictive perimembranous VSD with a predominantly left-to-right shunt (Figure 7), left ventricular (LV) hypertrophy (IVS 15 mm, posterior wall 12 mm), LV end-diastolic diameter 46 mm, LV end-systolic diameter 29 mm, LV mass 245 g, and moderate pulmonary hypertension (PASP 55 mmHg).
The possibility of surgical VSD closure was discussed, but it was decided not to proceed since the defect was small, there were no hemodynamic repercussions and episodes of endocarditis had been infrequent – one at age 18 and another 31 years later.
The patient was discharged clinically well, and advised of the need for prophylactic therapy against infective endocarditis and for regular cardiological assessment.
DiscussionAs stated above, right-sided endocarditis mainly affects intravenous drug users, although it can also be associated with use of pacemakers or central venous catheters, cutaneous or gynecological infections or bacteremia in patients with congenital heart disease with left-to-right shunt. Septic pulmonary embolism is a common form of presentation.6
Physical examination10–13 may detect a murmur indicating tricuspid regurgitation (holosystolic, audible at the left lower margin of the sternum, intensifying on inspiration and diminishing with expiration and the Valsalva maneuver); while characteristic, this is found in less than half of patients. There may also be signs of right heart failure, including distended neck veins, congestive hepatomegaly and hepatojugular reflux. Staphylococcus aureus is the most common causal agent, being responsible for 50% of cases of right-sided endocarditis, while 20% are caused by streptococci or enterococci, and 10% by Gram-negative bacilli.10
Radiological findings can include triangular focal opacities in the lungs, sometimes with cavitation, corresponding to septic pulmonary emboli. The latter are a common complication of right-sided endocarditis. In the study by Lejko-Zupanc et al.,6 69% of patients presented pulmonary embolism in the acute phase. The authors found more severe tricuspid regurgitation in the early stage of the disease, which they put down to the existence of pulmonary embolism in the acute phase, which contributed to valve dysfunction, subsequently improving after resolution of the infection.
In-hospital mortality due to infective endocarditis has decreased over time, from 40-50% in the 1950s to 16-27% at present.
The incidence of infective endocarditis in intravenous drug users is 2-5% a year, with a recurrence rate of 30% if drug use is continued.
Right-sided endocarditis is generally less aggressive than left-sided, and prognosis in cases of tricuspid valve infection by Staphylococcus aureus is usually favorable, with a good response to medical therapy. Mortality is 4-5%.1,14
In conclusion, while uncommon, right-sided endocarditis can affect individuals with no history of drug abuse, and the possibility of a congenital heart defect should be borne in mind. In the case presented, the patient reached the age of 49 without his VSD being diagnosed, but the fact that he had had a previous episode of tricuspid valve endocarditis was an indication of this possibility.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Moura Gonçalves A, et al. Endocardite da válvula tricúspide em doente com cardiopatia congénita. Rev Port Cardiol 2012. http://dx.doi.org/10.1016/j.repc.2012.10.002