The authors report a rare clinical case of myocardial bridging of the three major coronary arteries, which manifested in an unusual way with severe biventricular dysfunction in the context of tachycardia. For the diagnosis, the authors relied on non-invasive multimodality cardiac imaging, including cardiac magnetic resonance, computed tomography angiography and myocardial perfusion scintigraphy. The implementation of targeted medical and neurohormonal therapy resulted in the recovery of ventricular function and clinical improvement.
Os autores descrevem um caso clínico raro de bridging miocárdico dos três territórios coronários, que se apresentou de forma invulgar com disfunção biventricular grave em contexto de taquicardia. Para o diagnóstico os autores basearam-se em exames de imagem cardíaca não invasivos, como a ressonância magnética cardíaca, a angiografia tomográfica computorizada e a cintigrafia de perfusão miocárdica. A instituição de terapêutica médica dirigida e neuro-hormonal permitiu a recuperação da função ventricular e a melhoria clínica.
Myocardial bridging is a congenital coronary artery abnormality, in which a portion of an epicardial coronary artery takes an intramuscular course,1 and was first described in 1736 by Reyman.2 The tunneled artery segment is compressed during systole and relaxes during diastole. The reported prevalence varies between 0.5% and 16% when assessed by coronary angiography, but in some autopsy series is as high as 80%.3
Nevertheless, despite its high prevalence, most patients are asymptomatic. However, the condition can manifest clinically as myocardial ischemia, acute coronary syndromes, coronary spasms, arrhythmias, left ventricular dysfunction and myocardial stunning, syncope or even sudden death.4
To date, there are only two cases in the literature that describe myocardial stunning caused by myocardial bridging, both related to the left anterior descending (LAD) artery.5,6
Myocardial bridges are most commonly located in the mid LAD artery.7 To date, there is only one published case of myocardial bridging involving three major coronary arteries.8
The development of new imaging techniques has improved the identification and functional evaluation of myocardial bridges, making it possible to link clinical presentation to systolic compression and hence commence therapy.9
Our aim was to present an extremely rare case of myocardial stunning, occurring in the context of myocardial bridging, involving three coronary arteries. Here, we highlight the significance of determining the cause behind myocardial dysfunction in order to select the most suitable therapy, as well as the importance of different imaging techniques to reach a diagnosis.
Case reportThe authors describe the case of a 36-year-old female teacher, diagnosed with dilated cardiomyopathy of unknown etiology established at 5 months of age, following growth retardation and the detection of a cardiac murmur. The patient was monitored by a congenital heart disease clinic up until adulthood and medicated with digoxin from 6 to 16 years of age. She was discharged from follow-up in 2007 in New York Heart Association (NYHA) class I and with an echocardiography only showing a mildly dilated left ventricle and preserved left ventricular ejection fraction (54% Simpson's biplane method).
She had no known cardiovascular risk factors, toxic habits or relevant family history, including heart disease at a young age or sudden death. She had an uneventful pregnancy at 28 years of age and has only ever been medicated with the contraceptive pill.
The patient presented to the Emergency Room of another hospital with symptoms of exertional dyspnea, palpitations and lower limb edema lasting one week. She reported that she had recently been experiencing high levels of stress at work, with high blood pressure values detected two months prior to presentation. As a result, she was started on propranolol, which she discontinued due to hypotension and dizziness.
On admission, the patient's blood pressure was 200/130 mmHg. She was tachycardic (120 bpm), afebrile, eupneic and had normal heart sounds. Expiratory time was slightly increased on lung auscultation and she presented with mild bimalleolar edema.
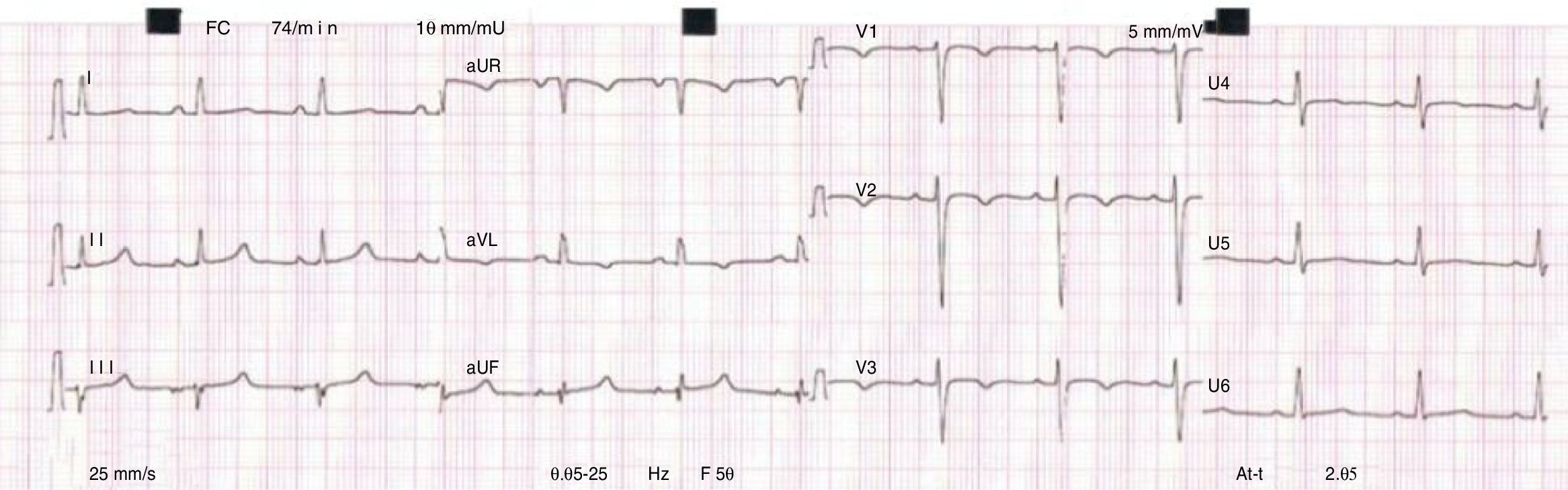
Electrocardiogram (ECG) revealed atrial tachycardia at 138 bpm, with left axis deviation and flattened T waves in leads I, aVL and V5-V6. A full blood count, coagulation test, renal function test and electrolytes were normal; high sensitivity troponin T and creatine kinase (CK) were normal; and mildly elevated transaminases (alanine transaminase: 75 IU/l and aspartate transaminase: 54 IU/l) and D-dimers were observed. Chest X-ray showed bilateral interstitial infiltrate, which was more pronounced at the lung bases, and an increased cardiothoracic ratio. A transthoracic echocardiogram revealed a non-dilated left ventricle with severe global systolic dysfunction (left ventricular ejection fraction [LVEF] 32%), a non-dilated right ventricle, moderate mitral and tricuspid valve regurgitation and an estimated pulmonary artery systolic pressure (PASP) of 50 mmHg. Two intracavitary masses were also seen and assumed to be thrombi.
The patient underwent a computed tomography pulmonary angiography, which revealed a repletion defect in the left ventricle apex and mild bilateral pleural effusion, with no other abnormalities.
Isosorbide dinitrate infusion was initiated, with good hemodynamic response. Anticoagulation with subcutaneous enoxaparin was also started.
The patient was then transferred to our site in less than 24 hours. During her hospital stay, we saw progressive clinical improvement and were able to initiate and uptitrate neurohormonal therapy with a beta-blocker, angiotensin-converting enzyme inhibitors and spironolactone. Ivabradine was also started to optimize heart rate control. Finally, warfarin was prescribed given the initial evidence of intracavitary thrombi.
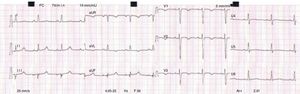
The ECG evolved spontaneously within the first 24 hours, with sinus rhythm, a heart rate of 74 bpm and inversion of the T waves in leads aVL and V1-V3 (Figure 1).
A repeat transthoracic echocardiogram showed a mildly dilated left ventricle, with diffuse hypokinesis (more pronounced at the interventricular septum and all the mid and apical segments of the remaining walls) and a severely reduced ejection fraction (LVEF of 18%). Moreover, an image compatible with a sessile thrombus at the apex and a pedunculated and mobile thrombus attached to the mid segment of the anterior septum were seen. Right ventricular (RV) dimensions were at the upper limit of normal and RV function at the lower limit of normal (fractional area change 34%). Furthermore, moderate mitral and tricuspid valve regurgitation and an estimated PASP of 46 mmHg were detected. The inferior vena cava was not dilated, with a 50% inspiratory collapse.
Laboratory blood tests were notable due to an increased of NT-proBNP value (3553 pg/ml on admission and 285 pg/ml at discharge). The remaining laboratory tests were normal, namely the full blood count; iron metabolism; renal function; electrolytes; C-reactive protein and erythrocyte sedimentation rate; glycated hemoglobin (HbA1c) and lipid profile; serial high-sensitivity T troponin and CK values; thyroid function; autoimmunity study and viral serology for HIV, and hepatitis B and C.
In order to rule out pheochromocytoma, a 24-hour urinary metanephrine assay was performed, with normal values.
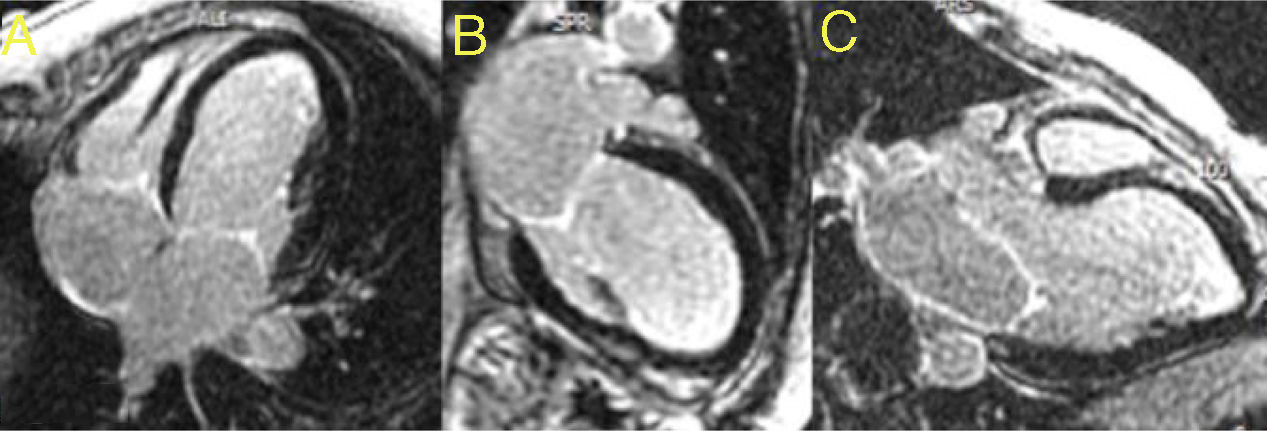
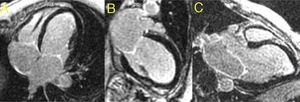
Cardiac magnetic resonance (CMR) imaging was performed to better characterize the myocardium and intracardiac masses. It confirmed severe biventricular systolic dysfunction (LVEF of 22% and right ventricular ejection fraction of 32%) and segmental wall-motion abnormalities. There was no evidence of signal hyperintensity suggestive of myocardial edema in T2-weighted images. In the early gadolinium enhancement images, no intracavitary thrombi were identified and, likewise, in the late gadolinium enhancement sequences, no areas of late enhancement were detected (Figure 2).
Following CMR imaging and during the patient's hospital stay, another transthoracic echocardiography was performed, confirming the disappearance of the left ventricular masses seen previously.
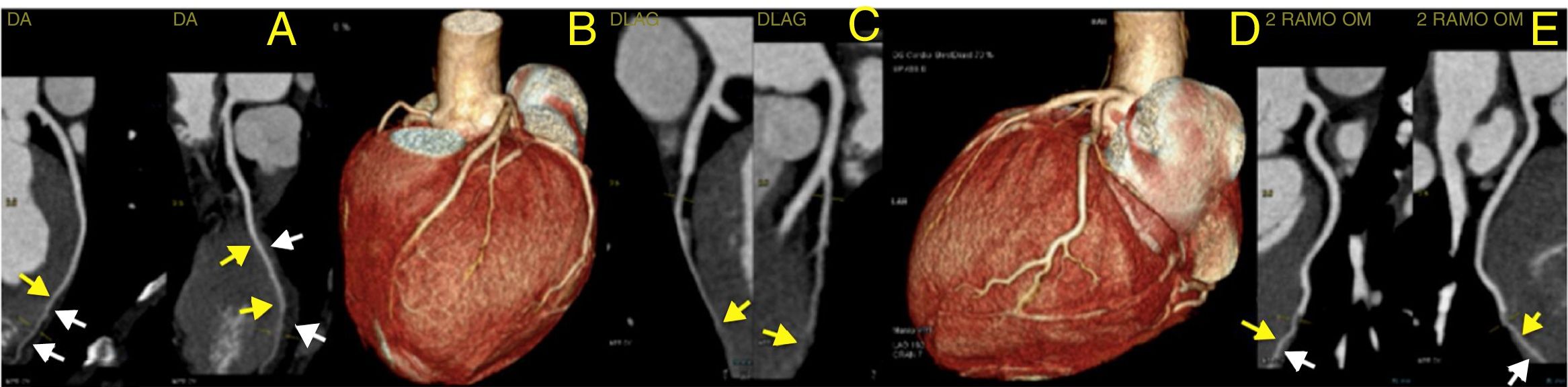
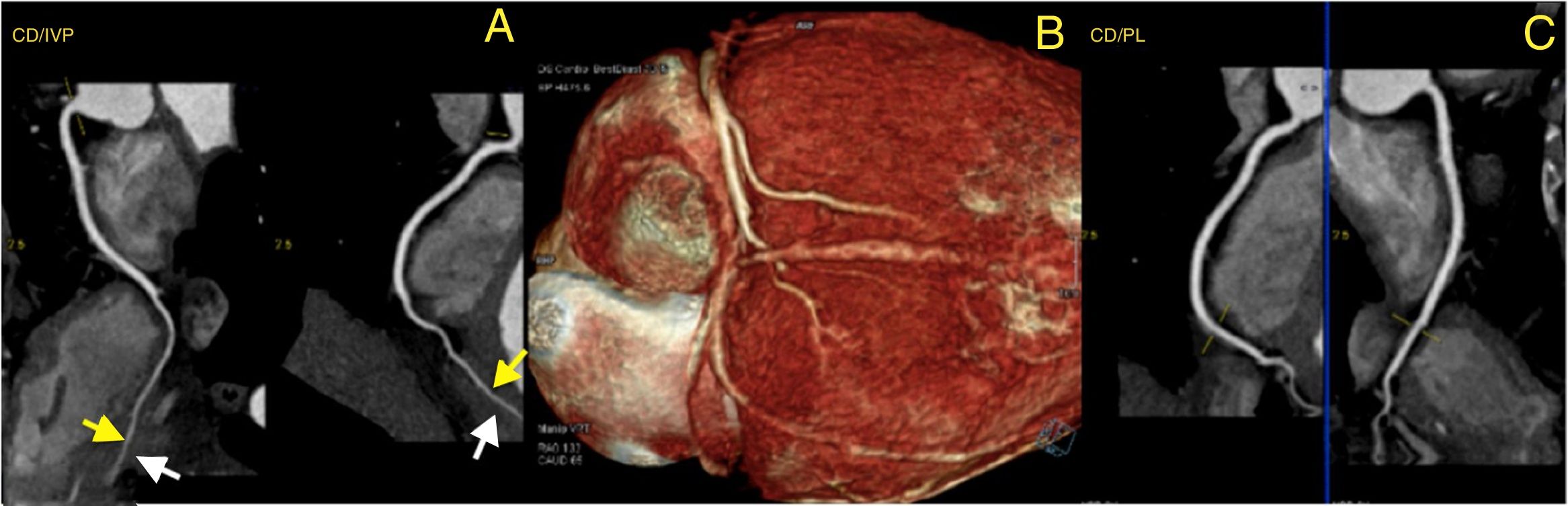
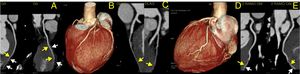
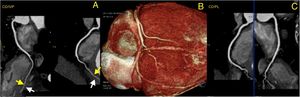
To rule out coronary artery disease (CAD), namely congenital coronary abnormalities, a coronary computed tomography angiography was requested. The latter revealed a calcium score of 0, the absence of atherosclerotic lesions and normal coronary ostia origins, with dominant right circulation. It also demonstrated a very marked intramyocardial route of the mid-distal segments of the LAD, diagonal and second obtuse marginal arteries (Figure 3) and distal segments of the interventricular posterior and postero-lateral arteries (Figure 4).
Computed tomography angiography: three-dimensional reconstruction of the heart showing coronary tree and bridging of left coronary arteries (panel B and D); curved multiplanar reconstruction showing very marked intramyocardial route of the mid-distal segments of left anterior descending artery (panel A), diagonal (panel C) and second obtuse marginal (panel E). Yellow arrows show intramyocardial coronary segments; white arrows show myocardial bridges.
Computed tomography angiography: three-dimensional reconstruction of the heart showing coronary tree and bridging of right coronary artery (RCA) (panel B); curved multiplanar reconstruction showing very marked intramyocardial distal segments of interventricular posterior artery (RCA/IVP) (panel A) and postero-lateral artery (RCA/PL) (panel C). Yellow arrows show intramyocardial coronary segments; White arrows show myocardial bridges.
In light of this result, we hypothesized that extensive coronary bridging might be the cause of ventricular dysfunction through myocardial stunning.
The patient was discharged for follow-up at the Heart Failure clinic in NYHA class II, on ramipril 5 mg once daily, carvedilol 25 mg twice daily, spironolactone 25 mg once daily, ivabradine 5 mg twice daily and warfarin.
One month after discharge, she underwent a myocardial perfusion scintigraphy, on optimal medical therapy, which revealed a stress-induced reduction of left ventricular systolic function (from 45% at rest to 39% during peak stress) and mild apical ischemia.
Four months after discharge, she went back to work and was in NYHA class I. Her NT-proBNP level was 199 pg/ml and the transthoracic echocardiography revealed an improvement in biventricular function with her left ventricle global systolic function at the lower limit of normal (biplane LVEF of 53%) and preserved right ventricular function.
DiscussionThe importance of this case stems from its rarity, being the second case described in the literature where bridging affects the three epicardial coronary arteries,8 and the third case of myocardial stunning in a myocardial bridging context.5,6 Moreover, it is the first case in which myocardial stunning occurred in three main coronary territories and sufficient biventricular dysfunction led it to be initially interpreted as a case of dilated cardiomyopathy and to manifest as NYHA class III congestive heart failure.
During the initial etiological investigation of this case, we investigated some of the most common causes of dilated cardiomyopathy among this age group and in patients with no cardiovascular risk factors, namely thyroid dysfunction, myocarditis, hypertension, toxic habits and infectious diseases, among others.
The fact that the patient's history of dilated cardiomyopathy dated back to early childhood did raise the possibility of a congenital cause, including coronary artery abnormalities, but since she was a young woman with no risk factors for CAD, and therefore a low probability for atherosclerosis, we decided to perform a coronary computed tomography angiography. Multislice spiral computed tomography defines bridges as segments surrounded by myocardium and is more helpful in identifying hemodynamically significant myocardial bridging. In invasive angiography, diagnosis depends on the change in diameter between systole and diastole within the bridged coronary segment.10 Although this malformation is present at birth, symptoms do not usually develop before the third decade of life, with the reason for this being unclear.3
In this patient, the coronary artery abnormality detected could be the cause of the myocardial dysfunction observed in infancy, through a myocardial stunning mechanism. Myocardial stunning is defined as prolonged yet reversible post-ischemic ventricular dysfunction, usually without tissue necrosis.11 This occurred in our patient who presented with ventricular dysfunction and serial negative troponin values, with no evidence of inflammation or scarring during CMR imaging, and with subsequent LV function recovery.
The pathophysiological mechanisms that contributed to the ischemic insult in this patient could be both her emotional stress-driven tachycardia and documented hypertension. Some authors have reported that tachycardia plays a major role in precipitating ischemic events in the presence of myocardial bridging5,10 by shortening the diastolic period – increasing the importance of systolic blood flow – and decreasing diastolic filling time and coronary flow reserve.2
In addition to the potential contribution of tachycardia to myocardial stunning in a coronary bridging context, the atrial tachycardia presented by this patient at the time of admission (the duration of which was unknown) may have been solely responsible for biventricular dysfunction – a phenomenon called tachycardiomyopathy. The possible mechanisms for myocardial dysfunction within this pathology are myocardial energy depletion, impaired energy utilization and myocardial ischemia, and it usually reverts after heart rate control.12
We have also considered Takotsubo cardiomyopathy13–15 in the differential diagnosis of our patient due to the pattern of the regional wall motion abnormalities and the presence of emotional stress. However, we did not detect any evidence of the myocardial lesions (edema or troponin elevation) that are usually present in this form of cardiomyopathy. Similarly, it was important for us to rule out pheochromocytoma, which can induce myocardial stunning directly or indirectly as a cause of tachycardia. In this case, urinary metanephrines were negative.
With regard to treatment, the nitrate infusion administered before the bridging diagnosis could potentially explain the initial decline in myocardial function (LVEF of 32% on admission, later estimated as 18%). Nitrates should generally be avoided because they increase the angiographic degree of systolic narrowing and can lead to an exacerbation of symptoms16 due to secondary tachycardia and hypercontractility from reflex sympathetic activation.17
In this patient, biventricular function was almost recovered after neurohormonal therapy. In myocardial bridging, heart rate control – in this case through beta-blocker and ivabradine use – allows a longer diastolic time, with consequent improvement of coronary perfusion. Beta-blockers and non-dihydropyridine calcium channel blockers are recommended as first-line drugs7 because, besides heart rate control, they also decrease myocardial contractility and coronary artery compression. Surgical myotomy, intracoronary stenting and coronary artery bypass graft surgery have been used for refractory symptoms, but the long term outcomes remain uncertain.7
Stress single-photon emission computed tomography can detect reversible myocardial perfusion defects in patients with myocardial bridging. The extent of ischemia is related to the degree of systolic luminal narrowing.3 In the reported patient, myocardial scintigraphy revealed a stress-induced decline in systolic function with mild apical ischemia, supporting the hypothesis that ischemia is behind the ventricular dysfunction.
In conclusion, this is the first reported case of myocardial stunning and severe biventricular dysfunction occurring in the context of three-vessel bridging. It also illustrates the importance of identifying a specific cause and mechanism of ventricular dysfunction in heart failure patients, in order to apply guided therapy and thus improve prognosis; in this case this was achieved through the use of multiple imaging modalities.
Conflicts of interestThe authors have no conflicts of interest to declare.