The aim of this study was to assess the value of tricuspid annulus myocardial isovolumic acceleration (IVA) in the assessment of right ventricular function in patients with acute pulmonary embolism (PE).
MethodsFifteen patients (mean age 60.6±11.3 years) with acute PE were enrolled and a control group was formed of 15 patients with a similar mean age (60.3±11.5). Patients who were diagnosed with acute PE by thoracic computed tomography angiography underwent transthoracic echocardiography at the time of diagnosis and at one month after diagnosis.
ResultsIn the control group IVA was 2.8±0.2 m/s2, while in the acute PE group, it was 2.0±0.1 m/s2 at the time of diagnosis and 2.9±0.1 m/s2 at the end of the first month. When IVA values of acute PE patients at the end of the first month were compared with their initial values and those of the control group, they had normalized (control and acute PE p<0.0001; control and PE at one-month follow-up p=0.983).
ConclusionIn our study, IVA was shown to be a reliable marker of right ventricular systolic function in patients with acute PE.
O objetivo deste estudo consiste em determinar o valor do tempo de aceleração isovulométrica miocárdica do anel tricúspide (IVA) na avaliação da função ventricular direita em doentes com embolia pulmonar (EP) aguda.
MétodosQuinze doentes (idade média 60,6 ± 11,3 anos) com EP aguda foram incluídos no nosso estudo e o grupo controle foi constituído por 15 doentes com idade média semelhante (60,3 ± 11,5). Após o diagnóstico de EP aguda por angiotomografia torácica computorizada, os doentes foram avaliados com ecocardiografia transtorácica, na altura do diagnóstico e no final do primeiro mês após o diagnóstico.
ResultadosEnquanto o valor do IVA foi de 2,8± 0,2 no grupo controle, no grupo com EP aguda foi 2,0± 0,1 na altura do diagnóstico e 2,9 ±0,1 no final do primeiro mês. Quando comparamos os valores de IVA dos doentes com EP aguda no final do primeiro mês com os valores iniciais e os valores do grupo controle, verificou-se que tinham normalizado (controle e EP aguda p <0,0001; controle e EP após um mês de seguimento p =0,983).
ConclusãoNo nosso estudo o IVA revelou-se um marcador adequado da função sistólica ventricular direita em doentes com EP aguda.
ejection fraction
isovolumic acceleration time
myocardial performance index
pulmonary artery pressure
pulmonary embolism
right ventricular
right ventricular ejection fraction
tricuspid annular plane systolic excursion
Pulmonary embolism (PE) is a relatively common cardiovascular emergency. It is difficult to diagnose and may be overlooked since it does not present with a distinctive clinical manifestation. However, since early treatment is extremely effective, an early diagnosis is of the utmost importance. Initial treatment basically involves re-establishing the blood flow through the obstructed pulmonary arteries and preventing an early recurrence, which may prove fatal.1
In more than 50% of hemodynamically stable patients with PE, no evidence of right ventricular (RV) dysfunction can be observed on transesophageal echocardiography.1,2 Severe RV dysfunction is seen only when hemodynamic collapse has occurred. In patients with echocardiographic evidence of RV dysfunction, the risk of mortality due to PE has been reported to be two times higher.1,2 However, there is still no consensus on the definition of RV dysfunction as determined by echocardiography.2
Recent studies have indicated that tricuspid annulus myocardial isovolumic acceleration (IVA) measured by tissue Doppler is a reliable parameter for assessment of RV systolic function independently of variations in pre- and afterload and that a lower IVA is an early marker of RV systolic dysfunction.3–5
The aim of this study was to assess the value of tricuspid annulus IVA, measured by transthoracic tissue Doppler echocardiography, and other widely accepted RV function parameters at the time of diagnosis and one month later, in the assessment of RV function in patients with acute PE.
MethodsPatients who presented to the emergency department of our hospital between February and October 2010 and were diagnosed with acute PE by thoracic computed tomography angiography were included in the study. Initially 19 patients with PE were included, but four of them were later excluded from the study since they were lost to follow-up. All the patients included in the study received thrombolytic treatment in standard doses after diagnosis. The control group consisted of eight males and seven females with no history of known cardiovascular disease or any systemic diseases other than hypertension or diabetes. The patients’ histories were recorded and a 12-lead electrocardiogram was obtained. Complete blood count, urea, creatinine, serum sodium and potassium levels and other laboratory parameters were tested. Patients in whom the quality of the transthoracic echocardiogram was inadequate due to poor echogenicity, those with pre-existing advanced mitral valve disease, and those with RV hypertrophy due to cor pulmonale were excluded from the study. Written informed consent was obtained from all patients. Demographic characteristics, etiological risk factors, diagnosis, and RV echocardiographic parameters at the end of the first month were recorded for each patient.
Transthoracic echocardiography and Doppler imagingBefore thrombolytic treatment was started, patients diagnosed with acute PE underwent standard transthoracic echocardiography in left lateral decubitus position using a Vivid 3 ultrasound system and a 2.5 mHz phased array transducer. Doppler recordings were made at 100 mm/s by synchronous single-lead electrocardiography. All measurements were obtained in three consecutive cycles, which were later averaged. All Doppler measurements were conducted at end-expiration so that the flow parameters were unaffected by respiration and were more consistent. The gain setting was set for optimum visualization of spectral view and the endocardial borders. Ejection fraction (EF) (to assess systolic function) and E-wave, A wave, E/A ratio, isovolumic relaxation time and deceleration time (to assess left ventricular diastolic function) were determined using tissue Doppler. Right ventricular ejection fraction (RVEF), as an indicator of RV systolic and diastolic function, was determined via two-dimensional RV fractional area change, two-dimensional RVEF, isovolumic relaxation time and tissue Doppler-derived tricuspid lateral annular systolic velocity (S¿). RV E wave, A wave, E/A ratio, RV shortening time, tricuspid annular plane systolic excursion (TAPSE), myocardial performance index (MPI), and IVA were also measured.6
Tricuspid annular plane systolic excursionTo determine tricuspid valve annular plane systolic excursion, an M-mode trace was recorded in apical 4-chamber view from the point where the tricuspid annulus joins the lateral free wall.6
Myocardial performance indexRV MPI was calculated based on the measurements from the tricuspid and RV outflow tract Doppler flow traces. The tricuspid flow Doppler trace was obtained with the sample volume of the pulsed wave Doppler at the tips of the tricuspid valve. In parasternal short-axis view, the sample volume was located immediately below the pulmonary valve so that the Doppler flow trace was obtained from the RV outflow tract. In pulsed Doppler recordings in apical 4-chamber view, the time elapsed from the end of the A wave until the end of the E wave was defined as the ejection time (MPI-a), while in the RV outflow tract pulsed Doppler recordings in parasternal short-axis view, the time elapsed from the beginning to the end of the systolic flow was defined as the ejection time (MPI−b).1,6 The following formula was used to calculate MPI: MPI=((MPI−a)−(MPI−b))/(MPI−b).
Isovolumic acceleration timeThe measurement was made by placing the pulsed tissue Doppler probe at the point where the tricuspid annulus joins the lateral free wall in apical 4-chamber view. IVA was calculated by dividing the peak isovolumic velocity, just before the peak of the R wave on the ECG and in front of the systolic wave on the tissue Doppler trace, by the time to peak velocity.1,6
Statistical analysisThe statistical analysis was carried out using SPSS version 16.1 (SPSS Inc., Chicago, IL, USA). Due to the small number of patients, non-parametric tests were employed. The Mann-Whitney U test was used for two independent variables, while the Kruskal-Wallis test was used for dependent variables. The results are expressed as means ± standard deviation and categorical variables as percentages. Statistical significance is based on a value of p<0.06.
ResultsDemographic and laboratory characteristics of the PE and control groups are presented in Table 1. No statistically significant differences in these parameters were observed between the two groups.
Patient characteristics.
| PE group (n=15) | Control group (n=15) | |
| Age (years) | 60.6±11.3 | 60.3±11.5 |
| Gender (female/male) | 7/15 | 8/15 |
| Hypertension (n; %) | 7 (46.7) | 8 (53.3) |
| Diabetes (n; %) | 4 (26.7) | 5 (33.3) |
| Smoking (n; %) | 6 (40) | 5 (33.3) |
| BMI (kg/m2) | 26.4±3.8 | 26.2±4.1 |
| SBP (mmHg) | 128.2±19.6 | 131.3±26.1 |
| DBP (mmHg) | 78.7±8.9 | 79.4±16.1 |
| Hemoglobin (g/dl) | 12.7±2.2 | 12.6±2.4 |
| Blood glucose (mg/dl) | 115±63 | 103±55 |
| BUN (mg/dl) | 28.3±10.4 | 23.6±12.4 |
| Creatinine (mg/dl) | 0.9±0.3 | 0.9±0.4 |
| Sodium (mmol/l) | 139.4±4.5 | 140.8±4 |
| Potassium (mmol/l) | 4.6±0.8 | 4.5±0.5 |
BMI: body mass index; BUN: blood urea nitrogen; DBP: diastolic blood pressure; PE: pulmonary embolism; SBP: systolic blood pressure.
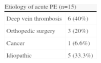
The etiologies of acute PE are summarized in Table 2, which shows that six patients (40%) had DVT, three (20%) had a history of orthopedic surgery, one (6.6%) had a history of cancer, and five (33.3%) had no risk factors.
The RV echocardiographic parameters of the control and PE groups are summarized in Table 3. RV end-diastolic and end-systolic volumes were significantly greater and RVEF was significantly lower in the PE group than in the control group. MPI, RV end-diastolic diameter, and pulmonary artery pressure (PAP) were significantly higher in the PE group than in controls, while TAPSE and IVA were significantly lower in the PE group.
Comparison of right ventricular echocardiographic parameters in the pulmonary embolism and control groups.
| PE group (n=15) | Control group (n=15) | p | |
| RVEDV (ml) | 76.3±10.4 | 49.2±11.8 | 0.011 |
| RVESV (ml) | 41.6±6.7 | 16.1±3.1 | 0.004 |
| RVEDD (mm) | 48.7±2.3 | 33.6±1.2 | 0.0001 |
| RVEF (%) | 48.3±3.9 | 64.8±2.9 | 0.001 |
| TAPSE (cm) | 16.7±1.2 | 29.8±0.5 | 0.0001 |
| MPI | 0.81±0.05 | 0.45±0.06 | 0.0001 |
| PAP (mmHg) | 53.5±4.1 | 18.6±4.8 | 0.0001 |
| IVA (m/s2) | 2.0±0.1 | 2.8±0.2 | 0.0001 |
IVA: isovolumic acceleration time; MPI; myocardial performance index; PAP: pulmonary artery pressure; RVEDD: right ventricular end-diastolic diameter; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; TAPSE: tricuspid annular plane systolic excursion.
Comparison of the echocardiographic parameters of the PE and control groups at the end of the first month is presented in Table 4. IVA, MPI, RVEF, and PAP had normalized, while TAPSE was greatly improved and was close to physiological values.
Comparison of echocardiographic parameters in the pulmonary embolism and control groups at the end of the first month.
| PE group (n=15) | Control group (n=15) | p | |
| RVEDV (ml) | 55.5±7.5 | 48.6±10.5 | 0.023 |
| RVESV (ml) | 24.5±4.1 | 15.1±3.7 | 0.018 |
| RVEDD (mm) | 38.3±2.2 | 33.2±1.8 | 0.01 |
| RVEF (%) | 55.1±2.9 | 61.8±2.2 | 0.505 |
| TAPSE (cm) | 23.3±0.9 | 28.7±0.9 | 0.002 |
| MPI | 0.68±0.08 | 0.51±0.11 | 0.07 |
| PAP (mmHg) | 29.6±6.7 | 21.3±4.1 | 0.222 |
| IVA (m/s2) | 2.6±0.2 | 2.7±0.3 | 0.283 |
IVA: isovolumic acceleration time; MPI: myocardial performance index; PAP: pulmonary artery pressure; RVEDD: right ventricular end-diastolic diameter; RVEDV: right ventricular end-diastolic volume; RVESV: right ventricular end-systolic volume; RVEF: right ventricular ejection fraction; TAPSE: tricuspid annular plane systolic excursion.
While mean IVA was 2.8±0.2 m/s2 in the control group, the value measured in the patients with acute PE was 2.0±0.1 m/s2. In the follow-up examination of these patients performed one month later, it was found to be 2.9±0.2 m/s2. The IVA values of the patients with acute PE had normalized at the end of the first month compared with the control group and their own values one month earlier (Figure 1).
DiscussionPE is one of the leading vascular emergencies; it is common, difficult to diagnose and can cause life-threatening acute RV failure due to obstruction of the pulmonary arteries. Since PE occurs frequently, has various clinical manifestations, and – most importantly – leads to high mortality, an early diagnosis is of the utmost importance. However, diagnosis is difficult and the condition may be overlooked since it does not present with a distinctive clinical manifestation. The main effects of a pulmonary thromboembolic episode are hemodynamic. Large and/or multiple embolisms may abruptly increase pulmonary vascular resistance to a level of afterload which cannot be matched by the right ventricle.1
RV function parameters are closely dependent on EF and tricuspid velocimetry, age, Doppler sample volume location and heart rate, as well as load and contractility.7,8 The complicated structure and asymmetrical shape of the right ventricle makes assessment of right heart function difficult.9 This has prompted the development of methods such as the non-geometric MPI, also known as the Tei index, and TAPSE.10–13 MPI is a non-invasive parameter that enables assessment of global systolic and diastolic ventricular function by Doppler.4,14 and is used in various clinical conditions including pulmonary hypertension, cardiomyopathy, complex congenital heart disease and obstructive sleep apnea. Its reliability and value have been demonstrated in numerous studies.14–18 TAPSE is related to RVEF and has also been shown to have prognostic value in patients with symptomatic heart failure and low EF.11,19 To the best of our knowledge, this study is the first to assess MPI and TAPSE in patients with acute PE. It indicates a decrease in TAPSE and an increase in MPI, both statistically significant, at the time of diagnosis, when patients with acute PE were compared to the control group. Although the one-month follow-up after the diagnosis showed an improvement in TAPSE and MPI, there was still a significant difference between the PE and control groups. We observed the same changes in RVEF, used to assess RV function, and other echocardiographic parameters, except for PAP.
In right-sided disease such as chronic obstructive pulmonary disease, RV infarction and pulmonary hypertension, pulsed tissue Doppler imaging indicates that RV systolic and diastolic function are affected.6,19 Although tissue Doppler is a new method, its applications are limited since myocardial velocities depend on volume load.20,21 Studies in recent years have demonstrated that IVA measured by tissue Doppler is a reliable parameter for assessment of RV systolic function independently of variations in pre- and afterload. Voegle et al. reported that RV IVA measured by tissue Doppler is a reliable parameter unaffected by volume load in assessment of RV contraction.5 In a study assessing RV systolic function in patients with mitral stenosis, Teyyareci et al. observed that IVA is negatively correlated with the Tei index and other classical parameters, and is significantly lower in patients with severe mitral stenosis. They also emphasized that it is a non-invasive and reliable alternative to determine the severity of mitral stenosis in patients without systemic findings of venous congestion.22 Tugcu et al. demonstrated that in patients with obstructive sleep apnea syndrome, although pulmonary and systemic arterial pressures are normal, of the echocardiographic parameters used to assess RV function only IVA shows a significant change. They associated this with subclinical RV dysfunction.23 Bayram et al. assessed RV function in 28 patients with obstructive sleep apnea syndrome undergoing continuous positive airway pressure therapy and observed a statistically significant improvement in IVA before and after treatment (before treatment: 4.1±0.5 m/s2; after treatment: 5.0±0.4 m/s2).24 In a study by Pauliks et al., a significant improvement in IVA was found in 39 patients with atrial septal defect before and after defect closure (before closure: 1.8±0.6 m/s2; after closure: 2.1±0.6 m/s2).25
Our study is the first to use IVA to assess RV function in patients with acute PE. We observed significantly lower IVA at the time of diagnosis of PE in comparison to the control group. During the follow-up examination one month later, we observed that IVA had normalized compared to the values from one month earlier. Also, when the patients with PE were compared to the control group one month after diagnosis, no statistically significant difference was seen between IVA values.
ConclusionMeasurement of RV function in acute PE is important to differentiate high- and low-risk groups in terms of prognosis. In our study, we assessed IVA and parameters widely used for assessment of RV function (RV end-diastolic and end-systolic volume, RVEF, PAP, RV end-diastolic diameter, TAPSE, and MPI), and reached the conclusion that RV tissue Doppler IVA is a reliable parameter for the assessment of RV systolic function in acute PE.
Study limitationsThe most important limitation of our study was the angle-dependent nature of pulsed tissue Doppler and the probable presence of artefacts. This means that inter-observer and intra-observer variability may be high. When obtaining measurements with routinely used transthoracic echocardiography devices, it is necessary to ensure that the device is optimally calibrated, and the procedure should be carried out carefully by experienced staff. Technological advancements in the equipment used for tissue Doppler imaging may reduce these limitations in the future. Finally, the number of patients in our study was only 15, and therefore further studies with a larger number of patients are needed to confirm our findings.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.








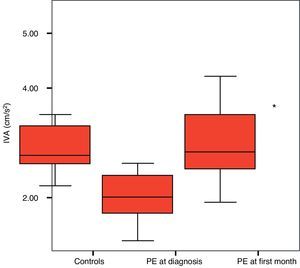 IVA values. *Control vs. acute PE p<0.0001; **control vs. PE at first month follow-up: p=0.983.'/>
IVA values. *Control vs. acute PE p<0.0001; **control vs. PE at first month follow-up: p=0.983.'/>

