There is an important link between platelets and inflammation, thrombosis, and vascular and tissue repair mechanisms. SCUBE1 (signal peptide-CUB-EGF domain-containing protein 1) may function as a novel platelet-endothelial adhesion molecule and play pathological roles in cardiovascular biology. Stent thrombosis (ST) following percutaneous coronary intervention is an uncommon and potentially catastrophic event that can manifest as myocardial infarction and sudden death. High platelet reactivity is a risk factor for thrombotic events, including late ST. For this reason, in the current study, we researched the role of SCUBE1 in the development of late coronary ST.
MethodsWe included 40 patients admitted to our hospital with a diagnosis of ST-elevation myocardial infarction (STEMI) and signs of late ST on a coronary angiogram. For the control group, we recruited 50 healthy gender- and age-matched individuals who were seen for health check-ups. We also randomly included 100 patients with a diagnosis of STEMI without ST.
ResultsThere were no significant differences between the groups in terms of baseline and demographic characteristics. The mean SCUBE1 level in patients with STEMI with late ST at admission and the STEMI without ST group was significantly higher than in the control group (p<0.01). The mean SCUBE1 level in the STEMI with late ST group was significantly higher than in the STEMI without ST group (p=0.03). In multivariate regression analysis, serum SCUBE1 (odds ratio [OR]: 1.022; 95% confidence interval [CI]: 1.011-1.033, p<0.001) remained an independent predictor for the presence of late ST. In addition, receiver operating characteristic curve analysis was used to determine the optimal SCUBE1 cut-off value for predicting late ST. The area under the curve was 0.972 (95% CI 0.95-0.98). The SCUBE1 cut-off value was 59.2 ng/ml, with a sensitivity of 95.4% and specificity of 82.9%.
ConclusionThe present work is the first clinical study to demonstrate that serum SCUBE1 levels are significantly higher in patients with late ST and serum SCUBE1 was an independent predictor for the presence of late ST in our study population.
Existe uma ligação importante entre plaquetas e inflamação, trombose e mecanismos de reparação tecidular e vascular. O biomarcador SCUBE1 [signal peptide-CUB (complement C1r/C1 s)-EGF (epidermal growth factor like domain-containing protein 1)] pode funcionar como uma nova molécula de adesão plaqueta-endotélio e pode ter papéis patológicos na biologia cardiovascular. A trombose de stent (TS) no seguimento de uma intervenção coronária percutânea é um evento incomum e potencialmente catastrófico que se pode manifestar como enfarte do miocárdio e morte súbita. A reatividade plaquetária elevada é um fator de risco para eventos trombóticos, inclusive a TS tardia. Por esse motivo, no presente estudo, investigámos o papel do biomarcador SCUBE1 no desenvolvimento da TS tardia nas coronárias.
MétodosIncluímos 40 doentes, admitidos no nosso hospital, com diagnóstico de enfarte do miocárdio com elevação do segmento ST (STEMI) e sinais de TS tardia na angiografia coronária. Para o grupo de controlo, reunimos 50 indivíduos saudáveis controlados para género e idade que foram submetidos a exames de rotina. Aleatoriamente incluímos também 100 doentes com diagnóstico de STEMI sem elevação do segmento ST.
ResultadosNão houve diferenças significativas entre os grupos no que se refere aos valores basais e às características demográficas. O nível médio do biomarcador SCUBE1 no grupo com STEMI e com elevação tardia de TS no momento do internamento e do grupo com STEMI sem elevação do segmento ST foi significativamente superior ao do grupo controlo (p < 0,01). O nível médio do biomarcador SCUBE1 no grupo de STEMI com TS tardia foi significativamente superior ao do grupo de STEMI sem elevação de ST (p = 0,03). Na análise de regressão multivariável, o nível sérico do biomarcador SCUBE1 [odds ratio (OR): 1,022; intervalo de confiança (IC) 95%: 1,011-1,033, p < 0,001] permaneceu como um fator preditor independente na presença de TS tardia. Além disso, a análise da curva ROC foi usada para determinar o valor cut-off do biomarcador SCUBE1 aprimorado para diagnóstico de TS tardia. O valor da área sob a curva (ASC) foi 0,972 (intervalo de confiança (IC) 95%: 0,95-0,98). O valor cut-off do biomarcador SCUBE1 foi 59,2 ng/ml com uma sensibilidade de 95,4% e uma especificidade de 82,9%.
ConclusõesO presente estudo é o primeiro estudo clínico que demonstra que os níveis séricos do biomarcador SCUBE1 são significativamente superiores nos doentes com TS tardia e que o nível sérico do biomarcador SCUBE1 é um fator preditor independente para a presença de TS tardia na população do estudo.
There is an important link between platelets and inflammation, thrombosis, and vascular and tissue repair mechanisms. Proliferative, mitogenic and inflammatory substances are released by activated platelets into the local microenvironment of vascular lesions.1 Thus, vascular and tissue repair mechanisms are affected and even controlled by activated platelets.
The epidermal growth factor (EGF) superfamily is a group of growth factors, cytokine-like mediators, and extracellular matrix proteins. A new gene for an EGF-related protein was isolated in 2002 in developing mice.2 This new mammalian gene encodes a protein with a signal peptide at the amino-terminus followed by several EGF (epidermal growth factor)-like repeats and one CUB (complement C1r/C1s) domain at the carboxyl terminus. This gene family was termed SCUBE, standing for signal-peptide-CUB-EGF-like domain-containing proteins. Expression of SCUBE1 (signal peptide-CUB-EGF-like domain-containing protein 1) has been detected in platelet-rich thrombi and in human atherosclerotic lesions. Tu et al.3 described experiments revealing that recombinant fragments of SCUBE enhanced platelet aggregation and adhesion. Activated and adherent platelets release SCUBE1, which adheres to the subendothelial matrix.4 Thus, SCUBE1 plays pathological roles in atherothrombosis by functioning as a novel platelet-endothelial adhesion molecule.5–7 Platelet activation is the most important step in arterial thrombosis, and is accordingly responsible for ischemic complications of acute coronary syndromes (ACS).8–10
Coronary stents are the mainstay of percutaneous coronary revascularization procedures, and their use has significantly decreased the rates of acute vessel closure and restenosis. Stent thrombosis (ST) after percutaneous coronary intervention (PCI) is an uncommon and potentially catastrophic event that can manifest as myocardial infarction and sudden death. Platelets play a pivotal role in thrombus formation, including ST, and thus optimal antiplatelet therapy is of critical importance in the prevention of ST.7 If the coronary stent is obstructed 30 days or more after implantation, it is termed late ST. Both bare-metal stents (BMS) and drug-eluting stents (DES) induce platelet activation, platelet adhesion and thrombus formation; therefore, effective antiplatelet therapy is mandatory for some time after stent implantation. Stents gradually become covered with endothelial cells that do not induce thrombus formation, and the need for platelet inhibition decreases. Cytotoxic drugs are used in patients with DES in order to reduce smooth muscle cell growth after PCI and to inhibit endothelialization.8 However, the underlying mechanism of late ST has not yet been fully elucidated.
High platelet reactivity is a risk factor for thrombotic events. For this reason, in this study, we examined the role of SCUBE1 in the development of late coronary ST.
MethodsThis study was approved by the institutional review boards of our hospitals, and written informed consent was obtained from the participants. The investigation conformed to the principles outlined in the Declaration of Helsinki.9
Study populationA total of 40 patients with STEMI who had evidence of late ST on a coronary angiogram were included in our study between March 2015 and December 2016. For the control group, we recruited 50 healthy gender- and age-matched individuals who were visiting the hospital for a health check-up. We also randomly included 100 patients who were admitted to our hospital with a diagnosis of STEMI without ST within the same date range.
Recruitment and exclusion criteriaSTEMI was defined according to the consensus of the American Heart Association/American College of Cardiology. Late ST was defined according to the Academic Research Consortium criteria.10
The exclusion criteria included significant valvular heart disease, idiopathic cardiomyopathies, any malignancy, rheumatologic and hematologic disease, renal failure (serum creatinine level >2.5 mg/dl) or liver disease (elevated aminotransferases). We also excluded potential mechanical causes of in-stent thrombosis (stent underexpansion, dissection and malapposition).
Plasma SCUBE1 assaysA full blood count was performed, and routine biochemical and cardiac enzyme assessments were conducted using blood specimens that were collected from the patient and control groups. In addition, 2 cc of blood was collected in citrate tubes from the control group and the study group (at first admission and just before discharge from the hospital) for investigation. Specimens were centrifuged for 15 min at 4000 g at 4°C. One cc of serum was placed in an Eppendorf tube and kept at -80°C until the assay was performed. Twenty-four hours before SCUBE1 assessment, the Eppendorf tubes were removed and kept at 4°C. Sera were gradually thawed over 24 hours, and SCUBE1 levels were measured after the samples had reached room temperature. An enzyme-linked immunosorbent assay kit was used to determine SCUBE-1 levels according to the manufacturer's instructions. Specimen absorbances were determined on a VersaMax tunable microplate reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm. The results were expressed in ng/ml.
Stent implantation procedureZotarolimus-eluting DES were deployed to treat in-stent lesions with standard techniques. During the procedure, patients received heparin to maintain an activated clotting time of ≥250 s. Heparin was not continued after the coronary stenting procedure. Before stenting, 180 mg of ticagrelor was administered orally to all study patients. If the thrombus burden was very high, intravenous glycoprotein IIb/IIIa inhibitor therapy was administered.
Quantitative coronary angiography analysisTwo experienced angiographers who were unaware of the study goal analyzed the angiographic results with an online quantitative angiographic analysis system. Percent diameter stenosis, minimal lumen diameter and reference diameter before and after stenting were measured during diastole after intracoronary nitroglycerin administration.
Statistical analysisSPSS version 20 (IBM SPSS) was used for the statistical analyses. The data were analyzed using descriptive techniques (mean and standard deviation). In addition, for quantitative data, the independent samples t test was used to compare groups of parameters that exhibited normal distribution, and the paired t test was used to determine the significance of the differences between the two matched groups. Categorical data were expressed as number and percentage. Pearson's chi-square test was used in the analysis of categorical data. The Mann-Whitney U test was used to compare nonparametric variables. We calculated that the enrollment of 140 patients with STEMI (n=40 with late ST and n=100 STEMI without ST) in the study group and 50 individuals in the control group would provide a power of 99.5 for demonstrating a significant difference in SCUBE1 levels between patients with late ST, patients without ST, and control subjects. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal SCUBE1 cut-off value for predicting late ST after primary PCI among patients with STEMI. Independent predictors for the presence of late ST were analyzed using logistic regression analysis. Possible confounding factors were tested using univariate regression analysis, and confounders with a p value of <0.25 were tested using multivariate logistic regression analysis. A p value of less than 0.05 was considered to be statistically significant.
ResultsThe baseline characteristics and laboratory parameters of patients and control subjects are shown in Table 1. There were no significant differences between groups in terms of age, gender, hypertension, diabetes, smoking, serum creatinine level or platelet count. There was no clopidogrel use in the control group or the STEMI without ST group. The number of patients who used clopidogrel in the STEMI with late ST group was 28, in whom the mean elapsed time from the deployment of the initial stent that became occluded was 84 days. The other 12 patients in the STEMI with late ST group received clopidogrel treatment for one month after discharge only. The Gensini score of the STEMI without ST group was 34±8.8, and that of the STEMI with late ST group was 36±10.2. The severity of coronary atherosclerosis did not differ between the STEMI groups (p=0.42). Thrombolysis in Myocardial Infarction (TIMI)-3 flow was measured in 32 patients after primary PCI in the STEMI with late ST group. TIMI-1 flow was observed in the other eight patients in this group. TIMI-3 flow was observed in all patients in the STEMI without ST group. The mean stent length in the STEMI with late ST group was 38±6.2 mm, and that in the STEMI without ST group was 34±3.2 (p=0.34). Bifurcation stents were not used in any group. Glycoprotein IIb/IIIa inhibitors were administered to 28 subjects in the STEMI with late ST group after revascularization because of high thrombus burden.
Baseline characteristics and laboratory test results of the study population.
| Parameters | Controls (n=50) | STEMI without ST (n=100) | STEMI with ST (n=40) | p |
|---|---|---|---|---|
| Age, years | 62±6.4 | 60±3.3 | 64±4.7 | NS |
| Female/male | 24/26 | 48/52 | 19/23 | NS |
| BMI, kg/m2 | 28.0±4.2 | 29.6±3.9 | 30.1±4.4 | NS |
| Glucose, mg/dl | 88.5±5.4 | 90.3±6.5 | 92.4±3.7 | NS |
| BUN, mg/dl | 12.0 (9-19) | 14.0 (6-26) | 14.0 (9-29) | NS |
| Creatinine, mg/dl | 0.7 (0.5-1.1) | 0.7 (0.5-1.2) | 0.8 (0.5-1.4) | NS |
| Uric acid, mg/dl | 7.7±1.0 | 7.5±1.1 | 7.4±1.3 | NS |
| TC, mg/dl | 240 (124-312) | 288 (196-374) | 280 (190-380) | NS |
| TG, mg/dl | 173.7±100.2 | 181.4±60.3 | 181.4±61.5 | NS |
| HDL, mg/dl | 45.3±11.1 | 46.5±11.6 | 44.5±11.2 | NS |
| LDL, mg/dl | 151.2±43.5 | 170.4±27.3 | 168.4±21.9 | NS |
| hs-CRP, mg/dl | 0.3 (0-0.8) | 0.4 (0-2.0) | 0.4 (0-2.0) | NS |
| Hb, g/dl | 13.4±1.0 | 13.1±1.0 | 13.7±2.0 | NS |
| Platelets, ×103/μl | 247.4±54.0 | 253.9±52.5 | 250.9±47.5 | NS |
| Diabetes, n | 14 | 29 | 15 | NS |
| Smoking, n | 26 | 49 | 27 | NS |
| Hypertension, n | 28 | 54 | 21 | NS |
| Mean Gensini score | - | 34±8.8 | 36±7.2 | NS |
| Mean stent length, mm | - | 29±4.8 | 30±6.2 | NS |
| Bifurcation stenting | - | - | - |
Values are mean ± SD or median (min-max).
BMI: body mass index; BUN: blood urea nitrogen; Hb: hemoglobin; HDL: high-density lipoprotein cholesterol; hs-CRP: high-sensitivity C-reactive protein; LDL: low-density cholesterol; ST: stent thrombosis; STEMI: ST-elevation myocardial infarction; TC: total cholesterol; TG: triglycerides.
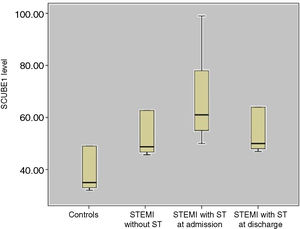
The mean SCUBE1 level was 30±7.4 ng/ml among the control subjects, 48.2±8.9 ng/ml in the STEMI without ST group and 67.6±6.9 ng/ml at admission and 50±7.6 ng/ml at hospital discharge in the STEMI with late ST group (Figure 1).
The mean SCUBE1 levels in the STEMI with late ST group at admission and in the STEMI without ST group were significantly higher than in the control group (p<0.01). The mean SCUBE1 level in the STEMI with late ST group was significantly higher than that of the STEMI without ST group (p=0.03). The mean SCUBE1 level in the STEMI with late ST group at discharge was significantly lower than that of the STEMI with late ST group at admission (p=0.04). The mean SCUBE-1 level of patients who had TIMI-1 flow after primary PCI was higher than that of patients who had TIMI-3 flow after primary PCI at discharge from the hospital in the STEMI with late ST group (66.1±4.4 ng/ml, 46±3.9 ng/ml, p=0.02) (Table 2).
Comparisons of mean SCUBE1 levels between the groups.
| Level in control group (30±7.4 ng/ml) | Level in STEMI patients without ST (48.2±8.9 ng/ml) | p<0.01 |
| Level in control group (30±7.4 ng/ml) | Level at admission in STEMI patients with late ST (67.6±6.9 ng/ml) | p<0.01 |
| Level at admission in STEMI patients with late ST (67.6±6.9 ng/ml) | Level in STEMI patients without ST (48.2±8.9 ng/ml) | p=0.03 |
| Level at admission in STEMI patients with late ST (67.6±6.9 ng/ml) | Level in STEMI patients with late ST at discharge (50±7.6 ng/ml) | p=0.04 |
| Level in STEMI patients with late ST and TIMI-1 flow after primary PCI (66.1±4.4 ng/ml) | Level in STEMI patients with late ST and TIMI-3 flow after primary PCI (46±3.9 ng/ml) | p=0.02 |
PCI: percutaneous coronary intervention; ST: stent thrombosis; STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction.
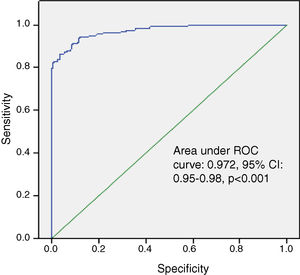
Univariate regression analysis showed that age and hemoglobin, platelet count, high-sensitivity C-reactive protein and SCUBE1 levels were possible confounding factors for the presence of late ST. In multivariate regression analysis, serum SCUBE1 (odds ratio [OR]: 1.022; 95% confidence interval [CI]: 1.011-1.033, p<0.001) remained an independent predictor for the presence of late ST (Table 3). In addition, ROC curve analysis was used to determine the optimal SCUBE1 cut-off value for predicting late ST. The area under the curve was calculated as 0.972 (95% CI 0.95-0.98). The optimal SCUBE1 cut-off value was 59.2 ng/ml, with a sensitivity of 95.4% and specificity of 82.9% (Figure 2).
Univariate and multivariate logistic regression analysis revealing independent predictors of late stent thrombosis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Age | 1.027 (0.991-1.064) | 0.140 | 1.018 (0.977-1.062) | 0.388 |
| Hemoglobin | 0.831 (0.631-1.094) | 0.187 | 0.940 (0.666-1.328) | 0.727 |
| Platelets | 1.004 (0.999-1.008) | 0.141 | 1.001 (0.995-1.007) | 0.672 |
| hs-CRP | 1.183 (0.943-1.484) | 0.146 | 1.039 (0.774-1.394) | 0.800 |
| SCUBE1 | 1.029 (1.019-1.039) | <0.001 | 1.022 (1.011-1.033) | <0.001 |
CI: confidence interval; hs-CRP: high-sensitivity C-reactive protein; OR: odds ratio.
The major findings of our study are as follows: (i) to the best of our knowledge, the present work is the first clinical study to show a graded relationship between late ST development and serum SCUBE1 level; (ii) for the first time, SCUBE1 was found to be an independent predictor for the presence of late ST.
SCUBE1 plays important roles in cardiovascular biology by activating platelets.4 SCUBE1 deposition has been immunohistochemically determined in the subendothelial matrix of advanced atherosclerotic lesions in humans. EGF-like repeats are responsible for adhesion.4 Platelet activation and aggregation are well recognized as primary reactions in arterial thrombosis and are thus involved in the ischemic complications of ACS.6,7 ACS are initiated by plaque rupture or erosion, with subsequent platelet activation and thrombus formation. The role of platelet activation in the pathogenesis of this atherothrombotic complication is shown by the importance of antiplatelet drugs in the management of ACS.9,10
Coronary stents are the mainstay of percutaneous coronary revascularization procedures, and their use has significantly decreased the rates of acute vessel closure and restenosis. ST after PCI is an uncommon and potentially catastrophic event that can manifest as myocardial infarction and sudden death. The optimization of stent implantation and dual antiplatelet therapy have markedly reduced the occurrence of this complication.11 DES were introduced into clinical practice to reduce the rates of restenosis that were observed with the use of BMS for the treatment of coronary artery disease.12
However, despite the promising results, concerns have been raised regarding the potentially increased risk of late ST, which is thought to be a consequence of delayed endothelialization.13 A number of studies have assessed the incidence of ST, most reporting a figure of 0.5-2%. Although a quantitatively minor problem, ST has a major clinical impact due to the high risk of myocardial infarction and death. The case fatality rate of ST has been reported to be as high as 45%.13 Delayed endothelial coverage, persistent fibrin deposition and ongoing vessel inflammation are associated with ST >30 days after stent implantation. Re-endothelialization after stent implantation is significantly delayed in patients with DES compared with BMS and is likely responsible for the higher rates of late ST associated with first-generation DES.14 Stent malapposition is defined as a lack of contact between the stent struts and the underlying arterial wall intima (despite full stent expansion at its nominal diameter). Late stent malapposition is typically the result of positive vessel wall remodeling (i.e., outward arterial wall expansion away from the stent struts that were well apposed at the time of implantation); this occurs more often in patients with DES than in those with BMS and has been associated with late ST.
Finally, neoatherosclerotic plaques can develop in the neointima within previously stented areas; these plaques may rupture and lead to ST.14 Neoatherosclerotic plaques have been observed in patients with BMS and DES. However, pathological evidence suggests that neoatherosclerosis may develop earlier in DES.15 Its development appears to be similar in patients with first- and second-generation DES.16
A study of 985 patients who underwent primary angioplasty for STEMI, including 102 patients with confirmed ST, demonstrated a higher occurrence of in-hospital death or recurrent myocardial infarction in patients who presented with ST compared with those presenting with STEMI secondary to a de novo lesion.17 Additionally, in patients with STEMI and ST, there was a higher thrombus burden, more frequent distal embolization and fewer successful results from PCI.18
Platelets play a pivotal role in thrombus formation, including ST, and so optimal antiplatelet therapy is of critical importance in the prevention of ST.7 High platelet reactivity is a risk factor for thrombotic events. Dai et al.19 demonstrated the role of SCUBE1 in patients with ACS and acute ischemic stroke. According to Wu et al.,20 plasma SCUBE1 appears to be redundant for baseline hemostasis but constitutes an important platelet adhesive system in pathological thrombosis. In their study, genetic loss or inhibition of plasma SCUBE1 protected mice against fatal thromboembolism and had a modest effect on bleeding. Hence, targeting plasma SCUBE1 could be a novel therapeutic strategy to prevent thrombosis.
Similarly, we found that SCUBE1 levels were very high in the study population at admission compared with control subjects (p<0.01). We also found that the mean SCUBE1 level in STEMI patients with late ST was significantly higher than in those with STEMI without ST (p=0.03). After coronary stent implantation and antiplatelet therapy, SCUBE1 levels decreased significantly (p=0.04). Mean SCUBE1 levels were high at hospital discharge because of eight patients in the STEMI with ST group who had TIMI1 flow on the coronary angiogram after primary PCI.
Our study results are consistent with findings from previous studies. We demonstrated for the first time a graded relationship between serum SCUBE1 level and late ST development. In multivariate regression analysis, serum SCUBE1 level was found to be a significant and independent predictor for the presence of late coronary ST.
Limitations of the studyFirst, our study population was small, and there is thus a need for future studies with larger sample sizes. Second, we had insufficient data regarding the types of stents that were completely occluded in the STEMI with late ST group.
Conflicts of interestThe authors have no conflicts of interest to declare.