Infantile hemangioma (IH) is one of the most common childhood tumors. There are various medical or surgical therapeutic options, all with suboptimal results. Recently, the successful use of propranolol for involution of IH was described. We report the results of a single-center experience with this therapeutic option.
ObjectiveTo prospectively assess the efficacy and safety of propranolol in children with infantile hemangioma.
MethodsWe performed a prospective analysis of clinical data of all patients with IH referred to a pediatric cardiology center for baseline cardiovascular assessment prior to propranolol therapy. Propranolol was given at a starting dose of 1 mg/kg/day and titrated to a target dose of 2–3 mg/kg/day according to clinical response. Efficacy was assessed through a photograph-based severity scoring scale. Safety was assessed by collecting data regarding significant side effects.
ResultsStarting in 2010, 30 patients (15 female) were referred for propranolol treatment of IH, at a median age of six months (1–63 months). The mean target propranolol dose was 2.8 mg/kg/day, with a mean duration of therapy of 12 months. All patients experienced significant reduction of IH size and volume. There were no side effects.
ConclusionsIn our experience propranolol appears to be a useful and safe treatment option for severe or complicated IH, achieving a rapid and significant reduction in their size. No adverse effects were observed, although until larger clinical trials are completed, potential adverse events should be borne in mind and consultation with local specialists is recommended prior to initiating treatment.
Os hemangiomas são a lesão tumoral cutânea mais frequente em idade pediátrica. Até ao momento todas as opções terapêuticas (tanto médicas como cirúrgicas) têm resultados sub-ótimos. Recentemente, foi descrita a utilização de propranolol para tratamento dos hemangiomas. Relatamos os resultados da nossa experiência com esta opção terapêutica.
ObjetivoAvaliação prospetiva da eficácia e segurança de propranolol em crianças com hemangioma infantil.
MétodosAvaliação prospetiva de todos os doentes com hemangioma referenciados para avaliação cardiovascular prévia ao início de terapêutica com propranolol. O propranolol foi administrado numa dose inicial de 1 mg/kg/dia e titulada para uma dose alvo de 2–3 mg/kg/dia, de acordo com a resposta clínica. A eficácia foi avaliada através de uma escala fotográfica. A segurança foi avaliada através da recolha de dados sobre os efeitos secundários significativos.
ResultadosDesde 2010, 30 crianças (15 do sexo feminino) com hemangiomas foram referenciados para avaliação cardiovascular prévia ao início de terapêutica beta-bloqueante, com uma idade média de 6 meses (1-63 meses). A dose alvo média atingida foi de 2,8 mg/kg/dia, com uma duração média de tratamento de 12 meses. Em todos os doentes se verificou uma redução significativa das dimensões e volume dos hemangiomas. Não foram observados efeitos colaterais.
ConclusõesNa nossa experiência, o propranolol é uma opção eficaz e segura para o tratamento de hemangiomas extensos ou complicados, obtendo-se uma redução rápida e significativa das suas dimensões. Não foram observados efeitos adversos contudo, recomenda-se a avaliação cardiovascular sistemática, prévia ao início de terapêutica com propranolol.
Infantile hemangiomas are the most common benign vascular tumors in infancy, affecting 5–10% of the population. Females are affected more often than males, with a ratio of 3:1.1 Typically they present shortly after birth, undergo a period of rapid proliferation, and then slowly involute over many years.2,3 Although most are small cutaneous vascular malformations of the face,4 they can also be large, disfiguring lesions with serious complications. Infants with large hemangiomas, especially those with a segmental distribution or hemangiomatosis, are at particular risk for extracutaneous complications. They may also be associated with other congenital malformations, such as PHACE,5 PELVIS4 or SACRAL syndrome.6,7
In most cases only parental education and reassurance are required. Although 85–90% of all infantile hemangiomas eventually undergo spontaneous involution, a minority can still cause disfigurement and serious complications, depending on their location (obstruction of airways and vision), size, and speed of regression, which can be associated with painful ulcerations and hemorrhage or even high-output heart failure.4,8,9 Hemangiomas with the potential to threaten a child's life or vital functions and those that ulcerate or cause substantial disfigurement warrant treatment,10 which may be medical or surgical, or a combination of both.11
At present there is no gold standard medical treatment. Unfortunately, current therapeutic approaches have limited success and significant adverse effects that limit their use.12–16 Since Léauté-Labrèze's accidental observation17 of the anti-proliferative effect of propranolol on infantile hemangiomas, propranolol has become increasingly popular18,19 as a successful therapeutic option, with fewer side effects than other treatments.
We present a single-center study describing the efficacy and safety of propranolol in children with infantile hemangiomas.
MethodsInclusion and exclusion criteriaAll patients were referred by their pediatric dermatologist or pediatric surgeon to a pediatric cardiology department for baseline cardiovascular assessment prior to propranolol therapy, and cardiovascular assessment during therapy.
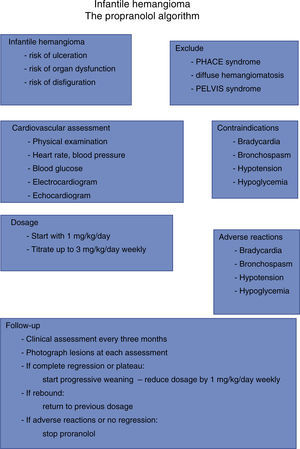
Patients with infantile hemangiomas were considered for propranolol treatment if they met the following criteria: eyelid involvement with risk of ocular occlusion or compression; airway obstruction; or large hemangioma with significant disfigurement or ulceration. Patients previously treated with other therapeutic modalities were also considered candidates for inclusion if the previous treatment had failed. Exclusion criteria included cardiac anomalies, central nervous system vascular anomalies as in PHACE syndrome, hypoglycemia, asthma or bronchospasm. While the study focused on infants, patients over one year of age were enrolled if their hemangiomas showed signs of continued proliferation or had shown no signs of resolution since infancy.
Treatment protocolAll patients underwent full cardiac examination before treatment, which included clinical examination, heart rate and blood pressure measurements, electrocardiogram, and echocardiogram. A baseline fingerstick blood glucose level was obtained. Further monitoring during the study included a weekly re-evaluation while the dose was being titrated to its maximum target dose, at weeks 1–4. Parents were instructed to look for signs of lethargy, poor feeding or wheezing. After the first month, monthly follow-up visits were scheduled until the end of the treatment for severity scoring of the hemangiomas, physical assessment and monitoring for adverse effects.
Dosage and durationPropranolol was given at a starting dose of 1 mg/kg/day, in two or three divided doses, and titrated to a target dose of 2–3 mg/kg per day according to clinical response.
Severity scoring systemA photograph-based severity scoring scale was used. Frontal and lateral pictures of every patient were taken before treatment and at every follow-up visit.
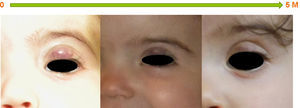
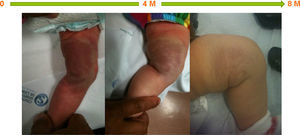
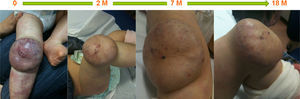
ResultsStarting in 2010 thirty patients were included in this study, of whom 15 (50%) were female; none were born prematurely. Superficial hemangioma was the predominant type, observed in 25 (83%) patients, whereas in three (10%) the hemangiomas had a segmental distribution (Table 1). The most frequent location was facial-cervical (70%). Propranolol was started at a median age of six months (1–63 months); the oldest patient was five years old. The mean target propranolol dose was 2.8 mg/kg/day (range 2.5–3.2 mg/kg/day). The mean duration of therapy was 12 months. All patients experienced immediate color changes and effects on the rate of hemangioma growth; in all patients a reduction of its size and volume was seen (Figures 1–3). No adverse effects were identified or reported by the patients or their parents. Propranolol was discontinued in five patients when the residual lesions ceased to respond to therapy; one had a slight relapse but it was not necessary to re-start propranolol.
Summary of patient characteristics.
| n=30 | |
| Female:male ratio | 1:1 |
| Type of hemangioma | |
| Superficial | 25 |
| Deep | 5 |
| Distribution of hemangioma | |
| Head | 8 |
| Periocular/eyelid involvement | 9 |
| Peribuccual involvement | 4 |
| Thoracic involvement | 5 |
| Limb involvement | 4 |
| Baseline assessment (blood pressure, fasting blood glucose, heart rate) | |
| Normal | All |
| Electrocardiogram pre-treatment | |
| Normal | All |
| Echocardiogram pre-treatment | |
| Normal | 24 |
| Abnormal | 6 |
In this group of patients propranolol therapy had similar effectiveness regardless of age at initiation of treatment (before or after six months of age). It was equally effective in hemangiomas considered to be beyond the proliferative phase. Also, segmental and nonsegmental, superficial, mixed, and deep infantile hemangiomas showed a similar response to propranolol (Figures 1–3).
DiscussionIn our experience propranolol appears to be a useful treatment for severe or complicated infantile hemangiomas, achieving a rapid and significant reduction in size. This reduction was mainly achieved during the first 20 weeks of treatment, and further treatment induced a less dramatic therapeutic effect. In this series of patients propranolol was equally effective in both segmental and nonsegmental infantile hemangiomas and in those beyond the proliferative phase.
Infantile hemangiomas have a predictable natural history.4,20 The majority are not present at birth. A hallmark of infantile hemangioma is its dramatic growth after birth, by diffuse proliferation of immature endothelial cells, followed by spontaneous regression. The majority of children with infantile hemangiomas require no treatment as the lesions regress over time and produce no long-term scarring; regression is complete in 50% of five-year-old patients and 90% of nine-year-olds.16 In approximately 10% of cases there can be serious or life-threatening hemangiomas, requiring treatment.10
Current treatment options for complicated hemangiomas include various medical or surgical modalities. Until recently, the mainstay of treatment for infantile hemangiomas was corticosteroids in various forms, including topical, intralesional, and oral formulations, the most common being oral prednisolone.12 Only in complicated or refractory hemangioma cases have other treatment modalities been considered, such as chemotherapeutic agents (vincristine, interferon-alpha), laser therapy, surgery or a combination of these, and, most recently, propranolol. Each treatment option has limited therapeutic benefit, with its own side effects and risks. However, in the past three years there have been more than 120 reports of the efficacy of oral beta-blockers, usually propranolol, as a highly effective therapeutic option for infantile hemangioma and its complications.18,21–23 Furthermore, it has been demonstrated that propranolol therapy is superior to oral corticosteroid treatment, the former standard therapy for infantile hemangioma, and should be considered the first-line agent given its safety and efficacy.24 Possible explanations for the therapeutic effect of propranolol on hemangiomas include vasoconstriction by decreasing the release of nitric oxide, which is immediately visible as a change in color, associated with palpable tissue softening. Other suggestions are down-regulation of proangiogenic signals such as VEGF, bFGF, MMP-9 and HBMEC, and induction of apoptosis in proliferating capillary endothelial cells.25,26 A large international randomized clinical trial is underway, but many are already advocating its use as a first-line treatment for infantile hemangiomas, in terms of both safety and efficacy.21,27 Its effects were first discovered by chance by Léauté-Labrèze et al. in 2008.17 Subsequent reports have emphasized propranolol's role not only in halting hemangioma growth but also in diminishing their size.21 Most groups have used a maximum target dose of 1–3 mg/kg/day.9,11,17,18,21,22,28–37 However, there are no established consistent protocols, particularly regarding the timing of treatment tapering and discontinuation.9,11,17–19,21,22,28–37 There is also no consensus regarding the relapse rate after discontinuation, with some studies showing no relapses,30–32 while others report minor recurrences.17,18,36 In our short series only one of the patients in whom propranolol was tapered showed a slight, non-significant, relapse, with no need to re-start treatment.
Until recently, the most common approach for patients with infantile hemangiomas in the post-proliferative phase was “active non-intervention”.38 In our series, propranolol improved esthetics in all patients, giving scope for more conservative surgical intervention in the future if necessary. These findings are consistent with those reported by Zvulunov et al.,39 Schupp et al.36 and Celik et al.,21 who reported the results of propranolol therapy for hemangiomas beyond the proliferative phase, and imply that oral propranolol therapy may be warranted in children with late residual infantile hemangiomas, prior to any surgical intervention.21 Still, the most impressive responses occurred in the youngest patients, a finding that is consistent with the natural history of hemangioma, in which 80% of its size is reached by six months of age, and justifies referring such cases earlier for optimal therapeutic response.
The appropriate monitoring protocol for assessment of adverse effects in infants with infantile hemangiomas, before and during propranolol treatment, has not been established.18,19 The potential side effects of beta-blockers, which are well known and include bradycardia, hypotension, and hypoglycemia,11,18,33,40–44 must be borne in mind. Propranolol is also contraindicated in patients with asthma, and it is not recommended during episodes of bronchiolitis.18,45 Notwithstanding, propranolol appears to be a safe drug when correctly administered. No adverse effects were observed in our series; none of our patients had symptoms of hypoglycemia or hypotension. However, until larger clinical trials are completed, potential adverse events should be borne in mind and consultation with local specialists such as pediatric cardiologists is recommended prior to initiating treatment. Patients with PHACE syndrome and severe cerebrovascular disease are, at least in theory, at risk for brain ischemia even with the relatively mild hypotension induced by beta-blockers.19 The risks and benefits in this subset of patients must be weighed carefully. Figure 4 summarizes our treatment algorithm based on our experience.
In conclusion, a better understanding of the mechanisms of propranolol-induced regression of infantile hemangiomas will provide opportunities to design even more successful therapies. Meanwhile, propranolol appears to be a uniquely effective and safe therapy for infantile hemangiomas, including in the post-proliferative phase, and should be considered the first-line therapy in this setting.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.