Childhood obesity is a worldwide health concern. Studies have shown autonomic dysfunction in obese children. The exact mechanism of this dysfunction is still unknown. The aim of this study was to assess the relationship between erythrocyte membrane fatty acid (EMFA) levels and cardiac autonomic function in obese children using heart rate variability (HRV).
MethodsA total of 48 obese and 32 healthy children were included in this case-control study. Anthropometric and biochemical data, HRV indices, and EMFA levels in both groups were compared statistically.
ResultsHRV parameters including standard deviation of normal-to-normal R-R intervals (NN), root mean square of successive differences, the number of pairs of successive NNs that differ by >50 ms (NN50), the proportion of NN50 divided by the total number of NNs, high-frequency power, and low-frequency power were lower in obese children compared to controls, implying parasympathetic impairment. Eicosapentaenoic acid and docosahexaenoic acid levels were lower in the obese group (p<0.001 and p=0.012, respectively). In correlation analysis, in the obese group, body mass index standard deviation and linoleic acid, arachidonic acid, triglycerides, and high-density lipoprotein levels showed a linear correlation with one or more HRV parameter, and age, eicosapentaenoic acid, and systolic and diastolic blood pressure correlated with mean heart rate. In linear regression analysis, age, dihomo-gamma-linolenic acid, linoleic acid, arachidonic acid, body mass index standard deviation, systolic blood pressure, triglycerides, low-density lipoprotein and high-density lipoprotein were related to HRV parameters, implying an effect on cardiac autonomic function.
ConclusionThere is impairment of cardiac autonomic function in obese children. It appears that levels of EMFAs such as linoleic acid, arachidonic acid and dihomo-gamma-linolenic acid play a role in the regulation of cardiac autonomic function in obese children.
A obesidade infantil constitui um problema de saúde a nível mundial. Alguns estudos têm demonstrado a presença de disfunção autonómica em crianças obesas. O mecanismo exato desta disfunção é ainda desconhecido. O objetivo deste estudo foi avaliar a relação entre os níveis dos ácidos gordos da membrana eritrocitária (AGME) e as funções autonómicas cardíacas nas crianças obesas, através do uso da variabilidade da frequência cardíaca (VFC).
MétodosUm total de 48 crianças obesas e de 32 crianças saudáveis foram incluídas neste estudo de caso-controlo. Dados antropométricos e bioquímicos, índices de VFC e níveis de AGME em ambos os grupos foram estatisticamente comparados.
ResultadosNas crianças obesas os parâmetros da VFC, que incluíam um desvio standard do intervalo normal-a-normal (NN), a diferença sucessiva do valor quadrático médio, o número de pares de NN sucessivos que diferem mais de cerca de 50 ms (NN50), a proporção de NN50 divididos pelo número total de NN, a potência de alta frequência e a potência de baixa frequência foram inferiores quando comparados com os do controlo, significando uma deficiência/insuficiência parassimpática. O ácido eicosapentaenoico e o ácido docosahexaenoico foram inferiores no grupo de crianças obesas (p < 0,001 e p = 0,012, respetivamente). Na análise de correlação do grupo obeso, o ácido linoleico, o ácido araquidónico, o desvio standard do índice da massa corporal, os triglicéridos, a lipoproteína de alta densidade mostraram uma correlação linear com parâmetros ≥ 1 da VFC e a idade, o ácido eicosapentaenoico, a pressão arterial sistólica e diastólica tiveram uma correlação com a frequência cardíaca média. Na análise da regressão linear, a idade, o ácido dihomo gama-linolénico, o ácido linoleico, o ácido araquidónico, o desvio standard do índice da massa corporal, a pressão arterial sistólica, os triglicéridos, as lipoproteínas de baixa e de alta densidades tiveram um efeito nos parâmetros de VFC implicando as funções autonómicas cardíacas.
ConclusãoHá uma insuficiência das funções autonómicas cardíacas nas crianças obesas. Parece que os níveis de AGME, tais como o ácido linoleico, o ácido araquidónico e o ácido dihomo gama-linolénico contribuem para a regulação das funções autonómicas cardíacas nas crianças obesas.
Childhood obesity is increasing worldwide and is associated with hypertension, increased insulin resistance, dyslipidemia and cardiovascular complications, resulting in high morbidity and mortality.1,2 It is known that altered energy metabolism due to an imbalance in the sympathoadrenal system has a crucial underlying role in the development of obesity. In addition, increased catecholamine secretion and altered reactions to stressors have been shown in obesity.3,4
Heart rate variability (HRV) is defined as beat-to-beat variability in heart rhythm and its measurement is a noninvasive and trustworthy method for assessment of cardiac autonomic function.4–6 Reduced HRV is associated with sudden cardiac death. Houlguin et al.7 reported that supplementation with omega-3 fatty acids caused a significant increase in HRV components in elderly individuals, which was associated with improved cardiac autonomic tone. In a meta-analysis of randomized, double-blind, placebo-controlled clinical trials, Mozaffarian et al.8 demonstrated that fish oil reduced heart rate, probably due to alterations in the automaticity or responsiveness of the sinus node and reductions in systemic vascular resistance. Although a few studies have reported impaired autonomic nervous system activity in obese children,5 there is no research showing the relationship between blood free fatty acids and HRV in the obese pediatric group. We aimed to assess cardiac autonomic function using HRV, as well as a possible relationship between HRV parameters and levels of erythrocyte membrane fatty acids (EMFAs), in obese children.
MethodsStudy populationThe study population consisted of 48 obese and 32 healthy non-obese children. The control group was assessed by means of a comprehensive medical history, physical examination (anthropometry, body mass index [BMI], blood pressure [BP]) and laboratory testing (whole blood count, kidney, liver and thyroid function tests, serum insulin and cortisol levels in terms of any syndromes, diseases, and drugs). Individuals with any disease associated with the heart, metabolism, liver, cardiovascular or respiratory systems, including arrhythmias, hypertension, or diabetes, acute or chronic infection, rheumatic, connective tissue or autoinflammatory disorders, hypothyroidism or with syndromes such as Prader-Willi, Laurence-Moon-Biedl or Cushing's syndromes, or taking any drugs affecting the autonomic nervous system such as beta-blockers or antiarrhythmics, were excluded from the control group. Patients with systolic ejection fraction <55% or with any congenital or structural heart disease on echocardiography were also excluded from the study. Informed consent was obtained from the children's parents for each procedure. All procedures in this study complied with the ethical standards of the Declaration of Helsinki. The study protocol and the patient information were approved by the ethics committee of Zekai Tahir Burak Women's Health Education and Research Hospital.
Standing height (in cm) to the nearest 0.1 cm using a Harpenden fixed stadiometer and body weight (in kg) to the nearest 0.1 kg using a SECA balance scale were measured with the children wearing only underwear and no shoes. BMI was defined as body weight in kg divided by the square of height in m. Children with BMI above the 97th and below the 85th centiles for age and gender according to the criteria of the International Obesity Task Force were classified as obese and healthy controls, respectively.9,10 Only pubertal children according to Tanner's criteria were included in the study.11
Heart rate variabilityTwenty-four-hour Holter electrocardiographic recording during regular daily activity was recorded using the 12-lead analog function of the Rozinn RZ152 digital Holter recorder (Rozinn Electronics, Inc., Glendale, NY, USA). The mean R-R interval (mean time for one beat in ms) and time domain analysis including standard deviation of all normal sinus R-R intervals (NN) for a selected period (SDNN), the standard deviation of the average NN intervals calculated over 5 min (SDANN), root mean squares of successive differences between adjacent NN intervals (RMSSD), the number of pairs of successive NNs that differ by more than 50 ms (NN50), the proportion of NN50 divided by the total number of NNs (pNN50), and power spectral analysis including the high-frequency (HF) component (0.15-0.40 Hz), an indication of cardiac vagal tone, the low-frequency (LF) component (0.04-0.15 Hz) reflecting baroreceptor activity, and the LF/HF ratio, representing sympathovagal balance, were used for HRV analysis. RMSSD and pNN50 mainly reflect parasympathetic tone in cardiac autonomic function. SDNN can be affected by sympathovagal and physiological changes.12
Laboratory testingSpecimen collection and storageVenous blood samples were obtained from each patient from the antecubital vein and stored in tubes containing K2-EDTA (purple top) and tubes containing clotting activator (red top). In red top tubes, blood samples were centrifuged immediately and serum samples were stored at -80°C until analysis. Red blood cell count and hematocrit levels of all whole blood samples were determined with an ABX Pentra XL 80 automated hematology analyzer (Horiba Medical, USA). Whole blood samples were then centrifuged for 10 min at 1500 g and erythrocytes were separated from the plasma. Plasma samples were stored in Eppendorf tubes freshly treated with butylated hydroxytoluene (BHT) at -80°C until analysis. Erythrocytes remaining in the tube were washed with saline. Briefly, instead of the plasma obtained from the blood, the sample was placed in an equal amount of saline and the supernatants were removed after the tubes were centrifuged for 10 min at 1500 g. This process was repeated twice. After the washing process, the erythrocytes were resuspended in saline to obtain a hematocrit of 45%. These erythrocyte suspension samples were stored, like the plasma samples, in BHT-treated Eppendorf tubes at -80°C until analysis.
Blood chemistry testsBlood chemistry parameters including fasting plasma glucose (after a fasting period of >12 hours), serum total cholesterol, high-density lipoprotein (HDL) and triglycerides were measured by enzymatic colorimetric methods with an Olympus AU2700 analyzer (Beckman Coulter, USA) using commercially available Olympus reagents. Low-density lipoprotein (LDL) levels were measured by the Friedewald equation. Plasma insulin levels were obtained with the electrochemiluminescence immunoassay method using an automated analyzer (Modular Analytics E170, Roche/Hitachi, Osaka, Japan). Impaired fasting glucose and dyslipidemia were defined as >95th percentile in healthy children in accordance with the guidelines.13,14 The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as HOMA-IR=fasting insulin (μU/ml)×fasting glucose (mg/dl)/405 in obese children. HOMA-IR values >2.67 and >2.22 for prepubertal boys and girls, respectively, and >5.22 and >3.82 for pubertal boys and girls, respectively, were accepted as indicating insulin resistance.15
Composition of erythrocyte membrane fatty acidsEMFAs were measured according to the method defined by Blau et al.16 and modified by Sertoglu et al.17
The principle of the gas chromatography assay is that fatty acid glycerol esters and plasmalogens are transmethylated by adding methanolic HCL to the sample and heated for 4 h at 90°C. After the sample is cooled, fatty acid methyl esters are extracted with hexane.
Erythrocyte suspensions were thawed at 4°C before the study and 50 μl of the internal standard solution, 50 μl plasma or erythrocyte suspension and 1 ml 3 N methanolic HCL were placed in 4 ml glass vials. The vial was closed and transmethylation proceeded at 90°C for 4 h. The vial was then removed from the oven and allowed to cool to room temperature and 2 ml of hexane was added to the vial, which was vortexed for 10 s. The upper layer (hexane) was transferred to a new glass tube and the hexane was carefully evaporated with nitrogen at room temperature. Finally, the residue was dissolved in 100 μl (plasma) or 80 μl (erythrocytes) of hexane.
An SPTM-2560 capillary column (100 m×0.25 mm×0.2 μm) was obtained for analysis and a TRACE GC Ultra gas chromatograph with a flame ionization detector was used (Thermo Scientific™, USA).
Statistical analysisThe statistical analysis was performed using SPSS for Windows, version 15 (SPSS Inc., Chicago, IL, USA). Normality of distribution was assessed by the Shapiro-Wilk test. Categorical variables and normally distributed continuous variables were compared with Fisher's exact test and the two-tailed Student's t test, respectively. For numerical variables, two-group comparisons were performed with the Mann-Whitney U test. Values for numerical variables were expressed as means ± standard deviation or mean. Categorical variables were depicted as numbers and percentage. Parameters affecting HRV were investigated using Pearson's correlation and linear regression analysis was used to determine independent predictors for each HRV parameter. A p value of less than 0.05 was considered as indicative of statistical significance.
ResultsA total of 48 obese patients – 28 (58.3%) male, mean age 11.95±2.42 years, and 36 healthy controls, 20 (55.5%) male, mean age 12.48±2.27 years – were included in the study (Table 1). There were statistically significant differences between the obese and control groups in BMI (p<0.001), BMI standard deviation (p<0.001), waist/hip ratio (p=0.016), systolic BP (p=0.020) and diastolic BP (p=0.008), but not in age (p=0.311) or gender (p=0.531).
Demographic, clinical and biochemical data of the obese children and controls.
| Variable | Control group (n=36) | Obese group (n=48) | p |
|---|---|---|---|
| Age (years) | 12.48±2.27 | 11.95±2.42 | 0.311 |
| Gender (male/female) | 20/16 | 28/20 | 0.531 |
| BMI (kg/m2) | 18.73±2.97 | 26.40±3.61 | <0.001 |
| BMI standard deviation | 0.83±0.52 | 2.15±0.50 | <0.001 |
| Waist-to-hip circumference | 0.83±0.05 | 0.89±0.12 | 0.016 |
| Systolic BP (mmHg) | 104.86±11.86 | 110.93±11.46 | 0.020 |
| Diastolic BP (mmHg) | 65.69±8.11 | 70.93±9.14 | 0.008 |
| Fasting glucose (g/dl) | 87.13±6.49 | 88.50±6.49 | 0.295 |
| Insulin (μU/ml) | 7.68±2.93 | 10.00±3.22 | 0.001 |
| HOMA-IR | 1.65±0.67 | 2.18±0.72 | 0.001 |
| Triglycerides (mg/dl) | 82.22±33.19 | 114.20±47.94 | 0.001 |
| Total cholesterol (mg/dl) | 164.83±28.37 | 165.62±35.20 | 0.912 |
| HDL cholesterol (mg/dl) | 54.61±10.81 | 45.77±8.83 | <0.001 |
| LDL cholesterol (mg/dl) | 93.75±24.34 | 100.08±23.91 | 0.237 |
BMI: body mass index; BP: blood pressure; HDL: high-density lipoprotein; HOMA-IR: homeostatic model assessment for insulin resistance; LDL: low-density lipoprotein.
Numeric values are presented as mean ± SD.
There were significant differences between the obese and control groups in terms of triglycerides (p=0.001), HDL (p<0.001), fasting insulin (p=0.001) and HOMA-IR (p=0.001), while no significant differences were found regarding fasting glucose (p=0.295), total cholesterol (p=0.912) or LDL cholesterol (p=0.237) (Table 1).
Erythrocyte membrane omega-6 fatty acid levels were as follows: linoleic acid (C18:2n6), arachidonic acid (C20:4n6) and dihomo-gamma-linolenic acid (C20:3n6), 48.37±9.64 μg/ml, 58.58±11.12 μg/ml and 6.69±2.24 μg/ml, respectively, in the obese group and 43.90±9.47 μg/ml, 60.31±10.27 μg/ml and 6.36±2.03 μg/ml, respectively, in the control group. Levels of these fatty acids in the obese group were not significantly different from those in the control group (p=0.053, p=0.485 and p=0.502, respectively).
Levels of erythrocyte membrane omega-3 fatty acids – icosapentaenoic acid (C20:5n3) and docosahexaenoic acid (C22:6n3) – were lower in the obese group (3.63±1.83 μg/ml and 10.96±5.62 μg/ml, respectively) than in the control group (16.89±3.46 μg/ml and 13.72±3.66 μg/ml, p<0.001 and p=0.012, respectively) (Table 2).
Levels of erythrocyte free fatty acids in obese children and controls.
| EMFA (μg/ml) | Control group (n=36) | Obese group (n=48) | p |
|---|---|---|---|
| Linoleic acid (C18:2n6) | 43.90±9.47 | 48.37±9.64 | 0.053 |
| Dihomo-gamma-linolenic acid (C20:3n6) | 6.36±2.03 | 6.69±2.24 | 0.502 |
| Arachidonic acid (C20:4n6) | 60.31±10.27 | 58.58±11.12 | 0.485 |
| Eicosapentaenoic acid (C20:5n3) | 16.89±3.46 | 3.63±1.83 | <0.001 |
| Docosahexaenoic acid (C22:6n3) | 13.72±3.66 | 10.96±5.62 | 0.012 |
EMFA: erythrocyte membrane fatty acid.
Numeric values are presented as mean ± SD.
Mean NN was 699.95±94.03 ms in the obese group and 752.16±72.41 ms in controls (p=0.007). The time-domain HRV parameters RMSSD, SDNN index, NN50 and pNN50 were lower in the obese group (61.43±28.94 ms, 69.12±24.03 ms, 22424.50±11600.56 and 24.96±14.04, respectively) than in the control group (84.08±38.37 ms, 86.13±27.32 ms, 29133.69±14255.94 and 34.84±14.38, respectively) (p=0.003, p=0.003, p=0.020 and p=0.023, respectively). LF and HF values were 26.61±6.37 normalized units (nu) and 17.41±10.23 nu in the obese, compared to 30.00±6.62 nu and 23.38±14.06 nu in controls (p=0.020 and p=0.028, respectively). Other HRV parameters including SDNN and SDANN among time-domain parameters and LF/HF ratio among frequency-domain parameters were similar in the obese and control groups (Table 3).
Parameters of heart rate variability in obese children and controls.
| Control group (n=36) | Obese group (n=48) | p | |
|---|---|---|---|
| Mean NN (ms) | 752.16±72.41 | 699.95±94.03 | 0.007 |
| Time domain parameters | |||
| SDNN (ms) | 148.55±44.92 | 129.18±45.08 | 0.054 |
| SDANN (ms) | 32.27±9.86 | 28.75±9.45 | 0.101 |
| RMSSD (ms) | 84.08±38.37 | 61.43±28.94 | 0.003 |
| SDNN index | 86.13±27.32 | 69.12±24.03 | 0.003 |
| NN50 | 29133.69±14255.94 | 22424.50±11600.56 | 0.020 |
| pNN50 (%) | 34.84±14.38 | 24.96±14.04 | 0.002 |
| Frequency domain parameters | |||
| LF (nu) | 30.00±6.62 | 26.61±6.37 | 0.020 |
| HF (nu) | 23.38±14.06 | 17.41±10.23 | 0.028 |
| LF/HF (nu) | 1.60±0.96 | 3.76±12.90 | 0.319 |
HF: high-frequency component; LF: low-frequency component; NN50: the number of pairs of successive NNs that differ by >50 ms; nu: normalized units; pNN50: the number of pairs of adjacent NN intervals differing by more than 50 ms divided by the total number of all NN intervals; RMSDD: root mean square of successive differences between adjacent NN intervals; SDANN: standard deviation of the average NN intervals in all 5-minute segments of the entire recording; SDNN: standard deviations of all NN intervals.
Numeric values are presented as mean ± SD.
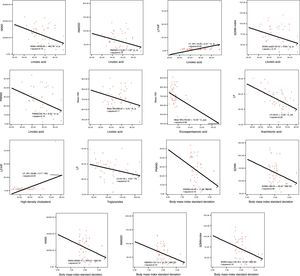
In Pearson correlation analysis in the obese group, linoleic acid, arachidonic acid, BMI standard deviation, triglycerides and HDL showed a linear correlation with one or more HRV parameter, and age, eicosapentaenoic acid, systolic and diastolic BP had a correlation with mean heart rate (Figure 1 and Tables 4 and 5).
Correlation analysis between heart rate variability parameters, dihomo-gamma-linolenic acid, docosahexaenoic acid, linoleic acid, eicosapentaenoic acid, arachidonic acid, body mass index standard deviation, triglycerides and high-density lipoprotein in the control group.
| SDNN | SDANN | RMSSD | SDNN index | NN50 | pNN50 | LF/HF | LF | HF | Mean NN | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | r | 0.147 | 0.044 | −0.137 | 0.058 | −0.196 | −0.189 | 0.493 | −0.050 | −0.333 | 0.084 |
| p | 0.393 | 0.799 | 0.427 | 0.736 | 0.251 | 0.271 | 0.002 | 0.773 | 0.047 | 0.627 | |
| Linoleic acid | r | 0.104 | 0.114 | 0.069 | 0.058 | −0.027 | 0.083 | 0.064 | −0.156 | −0.199 | 0.050 |
| p | 0.545 | 0.507 | 0.688 | 0.738 | 0.874 | 0.629 | 0.712 | 0.363 | 0.245 | 0.771 | |
| Dihomo-gamma-linolenic acid | r | 0.146 | 0.087 | 0.218 | 0.211 | 0.114 | 0.135 | 0.076 | 0.138 | −0.039 | −0.060 |
| p | 0.397 | 0.614 | 0.201 | 0.217 | 0.507 | 0.433 | 0.660 | 0.424 | 0.823 | 0.729 | |
| Eicosapentaenoic acid | r | 0.033 | −0.024 | 0.077 | 0.131 | 0.042 | 0.007 | 0.056 | 0.305 | 0.089 | 0.072 |
| p | 0.847 | 0.888 | 0.656 | 0.447 | 0.809 | 0.968 | 0.745 | 0.071 | 0.605 | 0.677 | |
| Docosahexaenoic acid | r | 0.142 | 0.027 | 0.139 | 0.188 | 0.032 | 0.090 | 0.065 | 0.307 | 0.172 | 0.135 |
| p | 0.408 | 0.874 | 0.417 | 0.273 | 0.852 | 0.600 | 0.705 | 0.069 | 0.316 | 0.433 | |
| Arachidonic acid | r | 0.006 | 0.038 | 0.134 | 0.023 | −0.073 | −0.167 | 0.063 | −0.058 | 0.059 | −0.149 |
| p | 0.970 | 0.826 | 0.435 | 0.895 | 0.671 | 0.330 | 0.716 | 0.738 | 0.733 | 0.387 | |
| BMI standard deviation | r | −0.302 | −0.143 | −0.041 | −0.246 | −0.130 | −0.091 | −0.310 | −0.260 | 0.029 | −0.419 |
| p | 0.077 | 0.411 | 0.814 | 0.154 | 0.456 | 0.604 | 0.069 | 0.132 | 0.871 | 0.012 | |
| Triglycerides | r | 0.492 | 0.437 | 0.284 | 0.398 | 0.046 | 0.359 | −0.085 | −0.235 | −0.106 | 0.322 |
| p | 0.002 | 0.008 | 0.094 | 0.016 | 0.788 | 0.031 | 0.622 | 0.167 | 0.540 | 0.055 | |
| HDL | r | −0.003 | −0.054 | 0.092 | 0.042 | 0.149 | 0.015 | 0.116 | 0.280 | 0.135 | −0.030 |
| p | 0.985 | 0.753 | 0.593 | 0.810 | 0.386 | 0.932 | 0.499 | 0.099 | 0.432 | 0.862 |
BMI: body mass index; HDL: high-density lipoprotein. Other abbreviations as in Table 3.
Correlation analysis between heart rate variability parameters, dihomo-gamma-linolenic acid, docosahexaenoic acid, linoleic acid, eicosapentaenoic acid, arachidonic acid, body mass index standard deviation, triglycerides and high-density lipoprotein in the obese group.
| SDNN | SDANN | RMSSD | SDNN index | NN50 | pNN50 | LF/HF | LF | HF | Mean NN | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | r | 0.011 | −0.208 | −0.056 | −0.060 | 0.081 | 0.066 | −0.070 | −0.026 | −0.061 | 0.428 |
| p | 0.942 | 0.156 | 0.704 | 0.686 | 0.583 | 0.657 | 0.638 | 0.858 | 0.684 | 0.002 | |
| Linoleic acid | r | −0.127 | −0.284 | −0.351 | −0.319 | −0.401 | −0.367 | 0.305 | −0.045 | −0.199 | −0.333 |
| p | 0.390 | 0.050 | 0.014 | 0.027 | 0.005 | 0.010 | 0.035 | 0.760 | 0.181 | 0.021 | |
| Dihomo-gamma-linolenic acid | r | 0.146 | 0.087 | 0.218 | 0.211 | 0.114 | 0.135 | 0.076 | 0.138 | −0.039 | −0.060 |
| p | 0.397 | 0.614 | 0.201 | 0.217 | 0.507 | 0.433 | 0.660 | 0.424 | 0.823 | 0.729 | |
| Eicosapentaenoic acid | r | −0.023 | −0.083 | −0.233 | −0.147 | −0.182 | −0.255 | 0.035 | 0.159 | −0.005 | −0.293 |
| p | 0.876 | 0.573 | 0.112 | 0.319 | 0.215 | 0.080 | 0.816 | 0.280 | 0.974 | 0.043 | |
| Docosahexaenoic acid | r | 0.142 | 0.027 | 0.139 | 0.188 | 0.032 | 0.090 | 0.065 | 0.307 | 0.172 | 0.135 |
| p | 0.408 | 0.874 | 0.417 | 0.273 | 0.852 | 0.600 | 0.705 | 0.069 | 0.316 | 0.433 | |
| Arachidonic acid | r | −0.242 | −0.241 | −0.097 | −0.225 | −0.155 | −0.061 | 0.091 | −0.512 | −0.014 | −0.016 |
| p | 0.098 | 0.099 | 0.513 | 0.124 | 0.291 | 0.683 | 0.541 | 0.000 | 0.928 | 0.914 | |
| BMI standard deviation | r | −0.293 | −0.214 | −0.367 | −0.319 | −0.339 | −0.405 | −0.073 | 0.045 | 0.094 | −0.247 |
| p | 0.043 | 0.145 | 0.010 | 0.027 | 0.018 | 0.004 | 0.623 | 0.762 | 0.530 | 0.090 | |
| Triglycerides | r | −0.204 | 0.079 | 0.199 | −0.016 | 0.051 | 0.116 | −0.227 | −0.311 | 0.194 | −0.137 |
| p | 0.165 | 0.593 | 0.174 | 0.916 | 0.731 | 0.431 | 0.120 | 0.032 | 0.192 | 0.354 | |
| HDL | r | 0.141 | 0.142 | 0.059 | 0.116 | 0.099 | 0.025 | 0.488 | 0.170 | −0.040 | −0.099 |
| p | 0.338 | 0.334 | 0.693 | 0.431 | 0.502 | 0.868 | 0.000 | 0.247 | 0.790 | 0.503 |
HDL: high-density lipoprotein. Other abbreviations as in Table 3.
In linear regression analysis, age, dihomo-gamma-linolenic acid, linoleic acid, arachidonic acid, BMI standard deviation, systolic blood pressure, triglycerides, low-density lipoprotein and HDL had a moderate effect on heart rate variability (Table 6).
Linear regression analysis of potentially influential factors including dihomo-gamma-linolenic acid, docosahexaenoic acid, linoleic acid, eicosapentaenoic acid, arachidonic acid, body mass index standard deviation, systolic and diastolic blood pressure, insulin resistance, triglycerides and high-density lipoprotein in the obese group in terms of cardiac autonomic dysfunction.
| HRV parameter | Variable | B | 95% CI | p | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| SDNN | Dihomo-gamma-linolenic acid | 5.49 | 0.08 | 10.90 | 0.047 |
| Arachidonic acid | −1.22 | −2.27 | −0.16 | 0.024 | |
| BMI standard deviation | −28.01 | −51.61 | −4.41 | 0.021 | |
| Systolic BP | 1.26 | 0.21 | 2.30 | 0.019 | |
| Low-density lipoprotein | −0.54 | −1.05 | −0.030 | 0.038 | |
| SDANN | Linoleic acid | −0.31 | −0.59 | −0.047 | 0.023 |
| Arachidonic acid | −0.24 | −0.48 | −0.01 | 0.043 | |
| RMSSD | Linoleic acid | −0.92 | −1.72 | −0.13 | 0.024 |
| BMI standard deviation | −18.92 | −34.31 | −3.53 | 0.017 | |
| SDNN index | Linoleic acid | −0.80 | −1.46 | −0.14 | 0.018 |
| Arachidonic acid | −0.63 | −1.20 | −0.06 | 0.030 | |
| BMI standard deviation | −14.35 | −27.00 | −1.71 | 0.027 | |
| NN50 | Linoleic acid | −475.99 | −787.35 | −164.63 | 0.004 |
| BMI standard deviation | −7079.82 | −13039.81 | −1119.84 | 0.021 | |
| pNN50 | Linoleic acid | −0.46 | −0.84 | −0.08 | 0.017 |
| BMI standard deviation | −10.22 | −17.51 | −2.93 | 0.007 | |
| LF/HF | Linoleic acid | 0.35 | 0.02 | 0.69 | 0.038 |
| High-density lipoprotein | 0.68 | 0.31 | 1.04 | 0.001 | |
| Arachidonic acid | −0.29 | −0.43 | −0.16 | 0.000 | |
| Triglycerides | −0.03 | −0.06 | −0.003 | 0.031 | |
| Mean NN | Age | 15.18 | 5.75 | 24.62 | 0.002 |
| Linoleic acid | −2.91 | −5.20 | −0.62 | 0.014 | |
| BMI standard deviation | −52.73 | −95.94 | −9.52 | 0.018 | |
| Low-density lipoprotein | −1.24 | −2.17 | −0.31 | 0.010 | |
BP: blood pressure; CI: confidence interval; HRV: heart rate variability. Other abbreviations as in Table 3.
To the best of our knowledge, this is the first study to investigate a possible relationship between EMFAs and HRV as an indicator of cardiac autonomic function in obese children. In this study, we determined that HRV parameters were decreased in obese children, implying impairment of sympathovagal balance and parasympathetic tone. We demonstrated lower erythrocyte membrane omega-3 fatty acids levels in obese children than in healthy children, and EMFA levels presented a correlation with one or more HRV parameters or with mean heart rate. In addition, we showed that EMFAs including linoleic acid, eicosapentaenoic acid and arachidonic acid, BMI, triglycerides, and HDL also had an effect on HRV parameters.
Various studies have been performed investigating autonomic function in obese children and adults. Piccirillo et al.18 reported impairment of sympathetic response in normotensive obese adults and Rossi et al.19 described decreased parasympathetic activity with normal sympathetic tone in obese adults. Zahorska-Markiewicz et al.20 detected increased sympathetic tone in obese adults, while Arone et al.21 found lower parasympathetic activity in obese adults, which was improved with weight loss. Rabbone et al.22 showed that adolescent obesity is characterized by imbalance in cardiac vagal response and sympathovagal tone and increased sympathetic hyperactivity. Similarly, the results of Taşçılar et al.5 indicate parasympathetic withdrawal and sympathetic predominance in obese children. Recently, Farinatti et al.23 showed that parasympathetic HRV indices were lower in obese children compared with healthy children. We also demonstrated sympathovagal imbalance and decreased parasympathetic tone, in agreement with the literature.
One of the best non-invasive techniques for assessing autonomic function is analysis of HRV parameters, the simplest of which is R-R interval (beat-to-beat) variability, and which provide numerical data on the autonomic nervous system.4 However, HRV parameters may be affected by many variables such as age, gender, ethnicity, nutrition, obesity, hyperlipidemia, diabetes, hypothyroidism, heart failure, hypertension, coronary artery disease, chronic obstructive pulmonary disease, renal failure, chronic liver disease and drugs.24 In our study, we excluded obese children with these risk factors to avoid possible confounding effects.
There is an association between insulin levels and HRV. HRV is lower in hypertensive patients with insulin resistance.22 Vinik et al.25 found that even mild hyperinsulinemia can impair circadian rhythms of autonomic activity. Recently, Hillebrand et al.26 reported a relationship between body fat and HRV and concluded that insulin resistance may decrease HRV in these patients. It is possible that visceral obesity increases free fatty acid levels in the portal circulation and reduces hepatic insulin clearance, resulting in hyperinsulinemia.27 Our patients had no insulin resistance but HOMA-IR and fasting insulin levels in the obese group were significantly higher than in controls. However, we found no correlation between HOMA-IR, fasting insulin, and HRV parameters in the obese group, and insulin levels were not predictive of HRV parameters in our study.
It is known that hyperlipidemia and increased plasma free fatty acid levels are common in the obese population and that infusion of free fatty acids into the portal vein can activate the sympathetic nervous system.28,29 Verwaerde et al.30 reported that a sudden increase in plasma free fatty acid levels caused tachycardia due to deterioration of parasympathetic tone in dogs, implying that free fatty acids play a significant role in autonomic activity. Shaltout et al.31 found a significant increase in the LF/HF ratio due to high plasma nonesterified fatty acid levels in rats, indicating a reduction in the vagal component of cardiac autonomic control. Stepniakowski and Egan32 assessed venous distensibility in 58 subjects including obese and lean hypertensives and normotensives. They concluded that obesity reduced venous distensibility, which might have hemodynamic effects. They also assessed dorsal hand vein responses to co-infusion of Intralipid 10% and heparin, raising fatty acids locally, in normal volunteers. The infusion reduced hand vein distensibility, implying that elevated nonesterified fatty acid levels may have potent vascular effects. In our study, triglycerides showed a negative correlation with LF, and HDL presented a positive correlation with LF/HF in the obese group. In addition, LDL, HDL, and triglycerides were risk factors for at least one HRV parameter. In view of these findings, we can state that blood lipid levels in obese children may contribute to sympathovagal imbalance.
Essential fatty acids such as omega-3 and omega-6 play important physiological roles in the cell membrane. It has been shown that lower omega-3 fatty acid levels and a decreased omega-3/omega-6 ratio are associated with various diseases.17,33,34 It has been reported that omega-3 fatty acids improved HRV variables in survivors of myocardial infarction.33 A study revealed that dietary supplementation with fish oil enriched with omega-3 enhances HRV indices and that there is a relationship between omega-3 concentrations in the cell membrane and HRV parameters.7 It has been suggested that omega-3 fatty acids prevent ventricular arrhythmias and sudden cardiac death due to myocardial ischemia by binding to sodium channels, and that omega-3 fatty acids reduce levels of tumor necrosis factor-alpha, interleukin-1 and interleukin-2, which are secreted in the early stage of myocardial ischemia and impair myocardial contraction and increase myocardial damage and reactive oxygen species.33 In addition, Minami et al.35 demonstrated that dietary docosahexaenoic acid increased levels of hippocampal acetylcholine, a vagal neurotransmitter, and prevents imbalance of parasympathetic tone by suppressing proinflammatory cytokines such as tumor necrosis factor-alpha and interleukin-1β, -6, and -18. Thus, omega-3 free fatty acids have protective effects on myocardial tissue by inhibiting proinflammatory cytokines and on cardiac function via the parasympathetic nervous system. In our study, linoleic acid and eicosapentaenoic acid had a negative correlation with HRV parameters reflecting parasympathetic tone. Arachidonic acid was also negatively correlated with LF, indicating impairment of vagal activity. Moreover, dihomo-gamma-linolenic acid, arachidonic acid and eicosapentaenoic acid had a moderate effect on HRV parameters. Although these findings may have been affected by the relatively small study population, specific features of the patients and possible confounding variables on HRV indices, the outcomes suggest a possible contribution of omega-3 and omega-6 fatty acids to decreased HRV in obese children.
Study limitationsThere are some limitations in this study. First, the study population is relatively small. Second, prepubertal children were not included. Third, we could not investigate the effect of EMFAs on cardiac systolic and diastolic function by advanced echocardiographic techniques. Finally, from a statistical standpoint, this study lacks the power to assess causality, and there is the risk of spurious correlations between variables because of multiple statistical testing.
ConclusionIn conclusion, we found impairment of cardiac autonomic function in obese children. Although the exact mechanism of this impairment is still unknown, omega-3 and omega-6 fatty acids contributed to autonomic dysfunction in these obese children. Further studies with larger patient populations are needed to define the precise effect of free fatty acids on cardiac autonomic function in obese children.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures in this study were performed according to the regulations of the ethics committee that approved this study and the Declaration of Helsinki. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial supportThe authors declare that this study received no financial support.
Conflicts of interestThe authors have no conflicts of interest to declare.