Our knowledge of the pathophysiology of heart failure (HF) underwent profound changes during the 1980s. Once thought to be of exclusively structural origin, HF began to be seen as the consequence of hormonal imbalance. A number of seminal studies were published in that decade focusing on the impact of neurohormonal activation in HF. Presently, eight neurohormonal systems are known to have a key role in HF development: four stimulate vasoconstriction and sodium/water retention (the sympathetic nervous system, the renin-angiotensin-aldosterone system [RAAS], endothelin, and the vasopressin-arginine system), while the other four stimulate vasodilation and natriuresis (the prostaglandin system, nitric oxide, the dopaminergic system, and the natriuretic peptide system [NPS]). These systems are strongly interconnected and are subject to intricate regulation, functioning together in a delicate homeostasis. Disruption of this homeostasis is characteristic of HF. This review explores the historical development of knowledge on the impact of the neurohormonal systems on HF pathophysiology, from the first studies to current understanding. In addition, the therapeutic potential of each of these systems is discussed, and currently used neurohormonal antagonists are characterized. Special emphasis is given to the latest drug approved for use in HF with reduced ejection fraction, sacubitril/valsartan. This drug combines two different molecules, acting on two different systems (RAAS and NPS) simultaneously.
Nos anos 80 assistiu-se a uma evolução profunda do conhecimento sobre a fisiopatologia da insuficiência cardíaca (IC): outrora considerada uma síndrome clínica de origem fundamentalmente estrutural, a IC começou a ser vista como a consequência de um desequilíbrio de forças hormonais opostas. Foi, de facto, nesta década que surgiram os estudos basilares sobre o impacto dos sistemas neuro-hormonais na IC. E destes, destacam-se oito: quatro de natureza vasoconstritora e anti-natriurética (o sistema nervoso simpático [SNS], o sistema da renina-angiotensina-aldosterona [SRAA], o sistema da vasopressina arginina e a endotelina), e quatro de natureza vasodilatadora e natriurética (o sistema das protaglandinas [PGs], do Oxido Nítrico [NO], o sistema dopaminérgico e o sistema dos péptidos natriuréticos [NPS]). Fortemente interligados entre si e com um sistema de regulação intrincado, estes sistemas funcionam habitualmente numa homeostasia delicada, cuja disrupção é sinal característico da IC. Nesta revisão é explorado o desenvolvimento histórico do conhecimento sobre o impacto destes sistemas neuro-hormonais na IC desde os seus primeiros estudos até ao conhecimento atual. Para além disso, são também revisitadas as oportunidades terapêuticas que cada um deles apresenta, bem como as famílias de antagonistas neuro-hormonais atualmente utilizadas na terapia da IC. Nesta última parte dá-se especial destaque ao último fármaco aprovado para utilização em doentes com IC com fração de ejeção reduzida, o sacubitril/valsartan, que combina dois antagonistas e que por isso atua simultaneamente em dois sistemas neuro-hormonais: o SRAA e o NPS.
Heart failure (HF) is a clinical syndrome of structural or functional origin characterized by deficient ventricular filling or ejection. Braunwald defines it as a condition in which the heart is unable to generate sufficient cardiac output to supply the body’s metabolic needs while maintaining normal venous return. The 2016 European Society of Cardiology (ESC) guidelines propose a new definition, terminology and diagnostic criteria for HF in the non-acute phase.1 Recent decades have seen profound changes in knowledge of HF, moving from the traditional view of the syndrome as of exclusively cardiac origin to the current integrated model in which the progressive nature of the disease is attributed to a complex series of structural and functional alterations, responses to which involve not only the cardiovascular system but also the renal, respiratory, neurohormonal, and other systems.2,3
Central to HF is myocardial contractility, which is determined by the series of events known as excitation-contraction coupling. Myofilament contraction is triggered by a rise in intracellular calcium (Ca2+), released from the sarcoplasmic reticulum following induction by Ca2+ (calcium-induced calcium release, CICR), or by the opening of voltage-sensitive channels (voltage-sensitive release mechanism, VSRM). In HF, the relative contributions of the CICR and VSRM pathways appears to be altered, the latter being weakened, which contributes to the contractile dysfunction that is characteristic of the syndrome.4
Of the pathophysiological mechanisms behind HF, here we focus on three: fibrosis (the accumulation of structural proteins in the extracellular matrix), apoptosis, and ventricular dysfunction. All three are related to an excessive inflammatory reaction that is typically found when cardiac muscle is under stress, such as in hypertension. In such situations, monocytes are activated, infiltrate into tissues and differentiate into macrophages. As a result, levels of pro-inflammatory cytokines rise, including tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, IL-6, IL-8 and monocyte chemoattractant protein 1 in the periphery and in the myocardium of patients with HF.5,6 These macrophages also produce transforming growth factor beta (TGF-beta), which is also associated with the myocardial fibrosis and remodeling typically seen in HF.5,7
In clinical terms, HF is manifested by a set of signs that include myocardial dilatation and hypertrophy, abnormal inotropism and raised heart rate, increased preload and afterload, and renal sodium/water retention.8 These alterations lead to respiratory difficulty, edema, crackles on auscultation, fatigue, abdominal distension, tachycardia, and other symptoms.9 The physical capacity of HF patients is usually limited and their quality of life is significantly affected.10
HF is a global public health problem with high costs for patients, hospitals and health systems. It is estimated that 23 million people worldwide have the condition.11,12 Although its incidence appears to have stabilized in recent years, its prevalence continues to rise, due to aging populations, improvements in treatment and hence increased survival in HF and other cardiovascular diseases, and increased prevalence of comorbidities.11 A recent systematic review by van Riet et al. estimated a median HF prevalence of 11.8% in those aged 60 years or over.13 In mainland Portugal, data published in 2002 indicate an overall prevalence of 4.36%, increasing markedly with age, from 1.36% in the 25-49 year age-group to 16.14% in those aged over 80 years.14 On the basis of these figures, HF is expected to affect 397 805 individuals in mainland Portugal in 2018 and 494 191 individuals in 2060.15
The risk factors for HF have been thoroughly studied and are well known. They include advanced age, male gender, myocardial ischemia, hypertension, diabetes, dyslipidemia, obesity and smoking.3,11 Curiously, the relative importance of these factors varies according to geographic region, with ischemic heart disease being the most important in western high-income countries.12
In 1989, our group, headed by Prof. Mário Cerqueira Gomes, published a review entitled “Neurohormonal mechanisms in heart failure: from pathophysiology to treatment”, which, although speculatively, set out to explain the benefits of the then emerging therapies for HF based on their favorable effects on activated neurohormonal systems.8 As well as being an homage to the pioneering vision of Prof. Cerqueira Gomes and his co-authors, some now no longer with us, the present review aims to revisit the impact of the neurohormonal systems in HF, from the discovery of the connection to the most recent evidence, as well as to examine the mechanisms through which current therapies act on and modify these systems. Special emphasis is given to a recently developed drug, sacubitril/valsartan, which is unusual in acting on two neurohormonal systems simultaneously, thereby weakening aggressive systems and strengthening protective systems in terms of their cardiovascular effects. This drug has been shown to have extremely promising efficacy and tolerability in several clinical trials, and an excellent review of its potential in the treatment of HF has been published in this journal.16
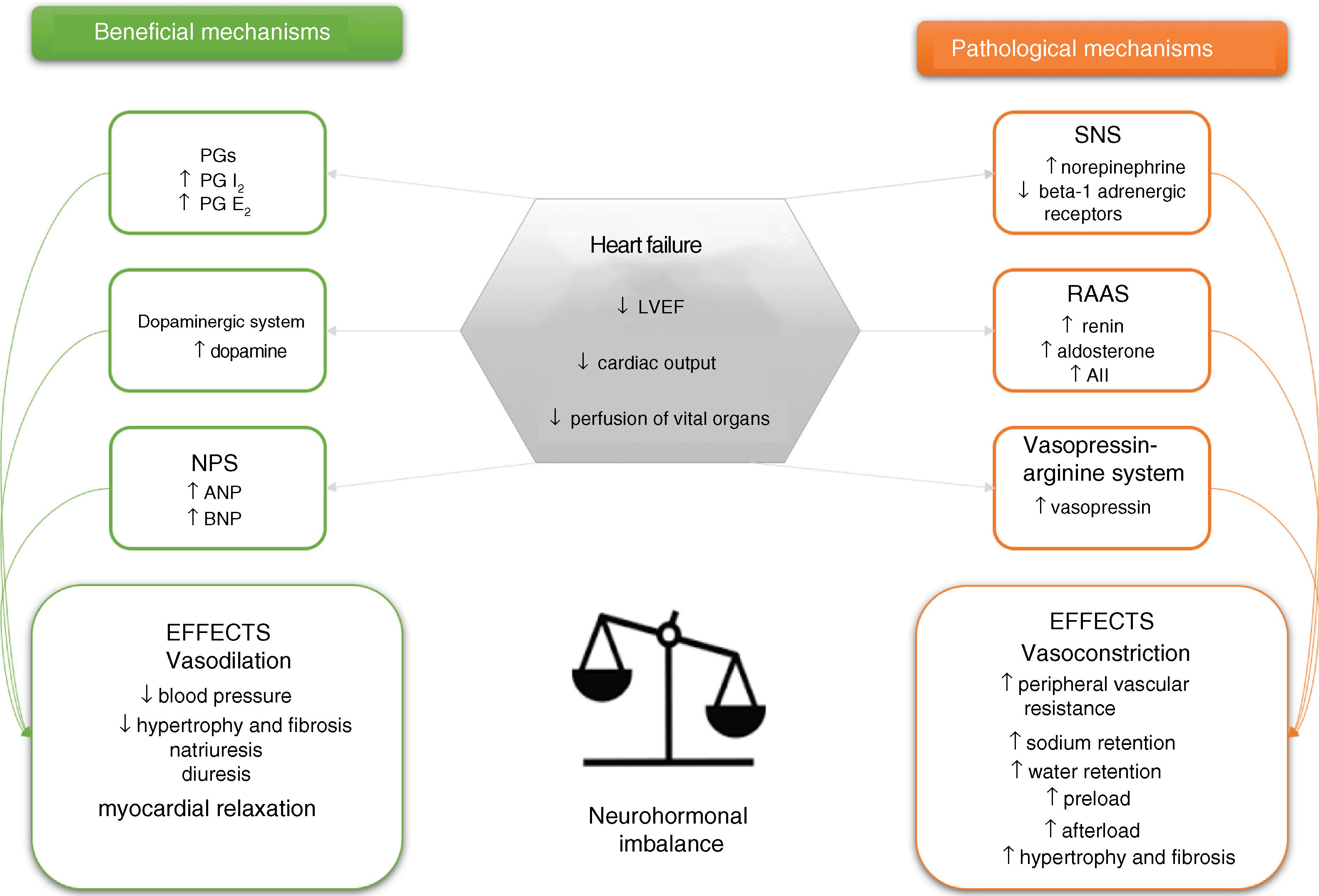
The impact of neurohormonal mechanisms in heart failureAs stated above, excessive stimulation of neurohormonal systems is linked with the pathophysiology of HF through autocrine and paracrine hormonal effects. Recognition of the importance of these systems dates back to the 1980s, when a series of studies produced growing evidence of the close connection between hormonal systems and the clinical setting and prognosis of HF.8,17,18 There are six neurohormonal systems involved in HF: three are mainly vasoconstrictor and antinatriuretic in function (the sympathetic nervous system [SNS], the renin-angiotensin-aldosterone system [RAAS], and the vasopressin-arginine system), while the other three stimulate vasodilation and natriuresis (the prostaglandin system and nitric oxide, the dopaminergic system, and the natriuretic peptide system [NPS]) (Figure 1).8,18
Schematic representation of the neurohormonal systems involved in heart failure and their main effects. ↑: increased; ↓: decreased; AII: angiotensin II; ANP: atrial natriuretic peptide; BNP: brain-type natriuretic peptide; LVEF: left ventricular ejection fraction; NPS: natriuretic peptide system; PG: prostaglandin; RAAS: renin-angiotensin-aldosterone system; SNS: sympathetic nervous system.
These systems are activated in the initial phase (compensation) of apparent adaptation to ventricular dysfunction and its systemic manifestations. However, continued stimulation, particularly of the vasoconstrictor and antinatriuretic systems, leads to the expression of their cytotoxic, procoagulant, proinflammatory and proproliferative effects, which play an important part in the progressive clinical worsening of HF.
Following the failure in the 1980s of treatments designed to stimulate cardiac inotropism, the strategy behind medical therapy in HF changed to counteracting the different vasoconstrictor and antinatriuretic neurohormonal systems and their effects. Among the therapies used for this purpose were combination therapy with hydralazine and nitrates,19 RAAS inhibition with angiotensin-converting enzyme inhibitors (ACEIs),20,21 antagonists of the AT1 receptor of angiotensin II (AII), commonly called angiotensin receptor blockers (ARBs),22 aldosterone antagonists,23 beta-blockers to inhibit the central nervous system,24 and simple reduction of heart rate with ivabradine.25 However, RAAS and SNS inhibition may not always be beneficial; rarely, patients even in the advanced stages of HF may suffer worsening of their disease if they take beta-blockers or RAAS inhibitors. This is presumably due to situations in which organ perfusion is highly dependent on RAAS activation or sustained cardiac inotropy, inhibition of which will be deleterious.
It is now clear that while inhibition of the SNS and RAAS and stimulation of the NPS reduce mortality and morbidity in congestive HF, no such benefit has yet been demonstrated for inhibition of vasopressin and stimulation of the prostaglandin-dopamine system. The simplest explanation for this is that the SNS, RAAS and NPS are phylogenetically more important for the organism to be able to respond to disturbances of volume and perfusion homeostasis, and therefore interactions between them have more marked effects. However, it is also possible that the currently available instruments for modifying these systems are more effective and selective than those available that affect the vasopressin and vasodilator prostaglandin-dopamine systems.
Sympathetic nervous systemThe SNS was one of the first systems to be studied in the context of HF.8 As early as 1897, Starling ascribed the tachycardia and vasoconstriction characteristic of HF to a reflex mechanism,26 while almost 70 years later, Chidsey and colleagues demonstrated that HF is linked to increased SNS activity, reflected in greater excretion of norepinephrine in HF patients, usually accompanied by reduced norepinephrine concentrations in the myocardium (Figure 2).26 It was subsequently demonstrated that plasma norepinephrine concentrations are directly correlated with left ventricular dysfunction,27 as long-term exposure to this hormone reduces the density of beta-adrenergic receptors (but not alpha-adrenergic or histamine receptors) in the myocardium of HF patients.28,29 The result is reduced inotropism and peripheral vasoconstriction, leading to increased afterload.
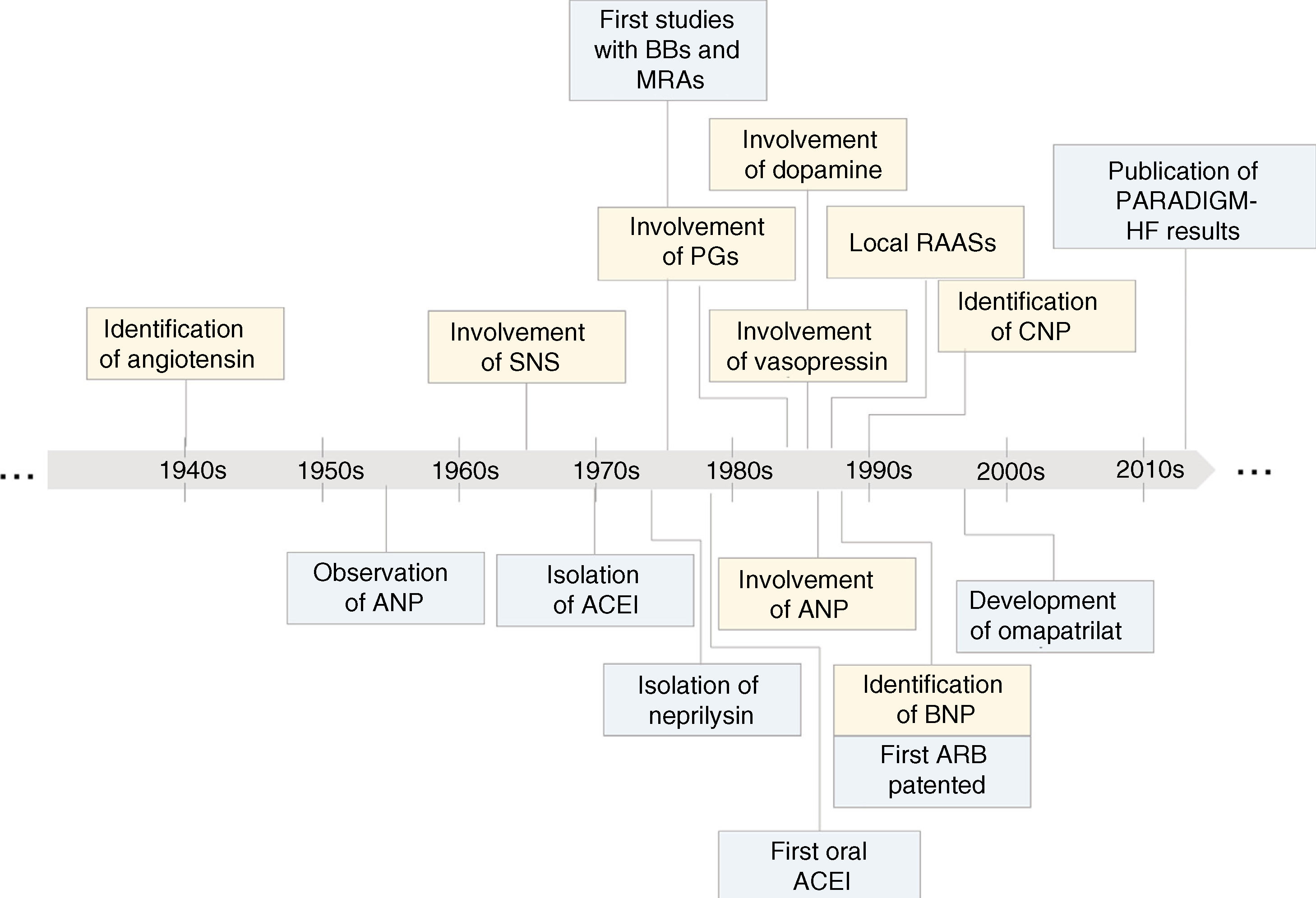
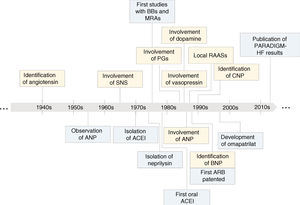
Key steps in the evolution of knowledge of the involvement of neurohormonal systems in heart failure (yellow) and of the development of treatments based on inhibition of these systems (blue). ↑: increased; ↓: decreased; ACEI: angiotensin-converting enzyme inhibitor; ANP: atrial natriuretic peptide; ARB: angiotensin receptor blocker; BBs: beta-blockers; BNP: brain natriuretic peptide; CNP: C-type natriuretic peptide; MRAs: mineralocorticoid receptor antagonists; PARADIGM-HF: Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure; PGs: prostaglandins; RAASs: renin-angiotensin-aldosterone systems; SNS: sympathetic nervous system.
The importance of the SNS in the cardiovascular system is now widely recognized, with effects including raised heart rate, positive inotropism and peripheral vasoconstriction. By contrast, the parasympathetic nervous system lowers heart rate through vagal innervation and baroreceptor activation.30 The action of these opposing forces, which regulates myocardial function via the sympathetic and parasympathetic nervous systems, is known as cardiac autonomic modulation.31 In HF, the SNS is continually stimulated by central and peripheral mechanisms, largely regulated by AII. This increased SNS activity is initially beneficial, increasing tissue perfusion pressure and counteracting the reduction in cardiac output, but in the long term it becomes pathological, with a range of repercussions resulting from increased norepinephrine levels, alterations in the neuronal hierarchy in the myocardium, and reduced density of beta-1 adrenergic receptors in cardiac tissue.30,32,33
There has been less research on the role of the parasympathetic nervous system in HF. It is known that parasympathetic stimulation is reduced, leading to a rise in heart rate and reduced heart rate variability, both of which are associated with increased mortality. Furthermore, stimulation of muscarinic receptors appears to have an antagonistic effect on beta-adrenergic stimulation.31
Renin-angiotensin-aldosterone systemInterest in the RAAS was awakened in 1898 when Tigerstedt identified a substance, which he called renin, produced by the renal cortex in rabbits that triggered a vasoconstrictor response.34 However, over 40 years were to pass before the substance directly responsible for the vasoconstriction, now known as angiotensin, was identified (Figure 2).35,36 On the basis of these and other landmark studies, the conventional picture of the RAAS emerged: renin produced in the kidneys catalyzes the transformation of angiotensinogen (produced in the liver) to angiotensin I, which in turn is catalyzed into AII by angiotensin-converting enzyme (ACE), produced in the lungs.
This view of the RAAS as functioning at a systemic level changed in the 1980s, when a series of experiments suggested the existence of local tissue RAASs (Figure 2).8,37 Angiotensin production has been demonstrated in different organs, including the myocardium, brain and kidney, and local AII levels may even exceed those in the plasma, performing important functions in terms of vascular resistance, cardiac function and adrenergic activity.8,37 In addition, several alternate enzyme pathways for angiotensin production have been identified, as well as different receptor types and subtypes, with a variety of effects on the cardiovascular system.
Briefly, the RAAS is activated in response to renal hypoperfusion, when baroreceptors in the wall of the renal afferent arteriole stimulate secretory granules to produce renin.38,39 This results in increased plasma renin activity (PRA), which is particularly noticeable in patients in New York Heart Association (NYHA) functional classes III and IV. However, in the less symptomatic functional classes I and II, and even with no significant rise in PRA, local RAASs may also be stimulated. There is thus systemic AII, which is the main mediator of the response to decreased volume and blood pressure, and local AII, which is responsible for the maintenance of organ and tissue homeostasis.8,39
AII has various effects, including increased peripheral vascular resistance, vasoconstriction of glomerular efferent arterioles, increased sodium/water reabsorption, vascular smooth muscle contraction, stimulation of aldosterone production by the adrenal glands, and enhanced production of prostaglandins (PGs).8,39,40 In the heart, AII stimulates myocyte and fibroblast hypertrophy, as well as collagen deposition, leading to myocardial fibrosis.8,39,40 This fibrosis and expansion of the extracellular matrix are two signs of the ventricular remodeling that is characteristic of HF with reduced ejection fraction (HFrEF).39 Similar effects are seen at the vascular level, including thickening of the arterial wall and endothelial dysfunction, as well as neurovascular effects, many of them mediated by aldosterone and other mineralocorticoid hormones. Finally, another effect of the RAAS is inactivation of bradykinin, a vasodilator peptide, through the action of ACE.40
The beneficial effects on HF symptoms and prognosis of drugs that modulate the RAAS are the strongest evidence of the importance of this neurohormonal system in the pathophysiology of the syndrome. There is a close relationship between the vasodilator effects of bradykinin and the effects of prostaglandins and nitric oxide (NO): the reduced vasodilation of small arterial beds dependent on endothelial NO production seen in HF is normalized by RAAS inhibition.41
Vasopressin-arginine systemThe first studies on the impact of vasopressin in HF date from the early 1980s, when researchers including Szatalowicz and colleagues in 1981 and Goldsmith and colleagues in 1983 made the discovery, almost simultaneously, that vasopressin levels were usually elevated in HF patients (Figure 2).42,43 The idea that this elevation could be related to the pathophysiology of HF arose a few years later,44 although vasopressin was still considered to be of lesser importance than the SNS and RAAS. In 1986, Creager et al., in a study monitoring the three systems – arginine-vasopressin, SNS and RAAS – in a cohort of 10 patients with HF found elevated vasopressin in only three of them.45 Nevertheless, these three patients responded to a vasopressin antagonist, presenting systemic vasodilation, thus proving that vasopressin does indeed contribute to the vasoconstriction seen in HF. Similarly, in the same year Johnston et al. published a study which demonstrated that a rabbit model of HF, although presenting normal vasopressin levels, had heightened sensitivity to the hormone,46 and administration of a vasopressin antagonist led to decreases in blood pressure and peripheral resistance as well as increased cardiac output.
Vasopressin is synthesized by the hypothalamus and stored in the posterior lobe of the pituitary gland. In physiological conditions, it is released in response to increased osmolarity or reduction in plasma volume, and its action – mediated by the V1a, V1b and V2 receptors – maintains homeostasis of water content and blood pressure.47 In HF, the SNS and RAAS trigger release of vasopressin in the absence of an increase in osmolarity.48 Vasopressin, in turn, stimulates vasoconstriction via the V1a receptor and increases water retention and urine concentration via the V2 receptor (and partly also the V1a receptor); it may also mediate the release of aldosterone via the V1b receptor.48,49 The overall result is increased vascular resistance and afterload, decreased irrigation and myocardial contractility, ventricular hypertrophy and remodeling, and water retention accompanied by pulmonary congestion, edema and increased preload.49 Furthermore, as a consequence of reabsorption of water by the kidneys associated with already low osmolarity, hyponatremia may occur, which is an important marker of poor prognosis and increased mortality in HF.47–49
EndothelinEndothelin (ET) is a peptide secreted by the endothelium that was discovered in 1988 by Yanagisawa et al.50 It is one of the most potent endogenous vasoconstrictors, its effects being mediated by binding to ETA receptors in vascular smooth muscle and myocardium and ETB receptors in the endothelium itself.51 Although usually produced in low quantities, levels rise in pathological conditions such as pulmonary hypertension, connective tissue disease, pulmonary fibrosis, and HF.52–55 Endothelin receptor antagonists include bosentan (non-specific), atrasentan (selective for subtype ETA), and tezosentan, which is used in hepatorenal syndrome and acute HF.
Vasodilator prostaglandins and nitric oxideIn 1984, Dzau and colleagues were the first to postulate that compensatory vasodilator systems could be activated in HF to counteract the vasoconstrictor effects of the SNS, RAAS and vasopressin-arginine (Fig. 2).56 They set out to analyze the involvement of vasodilator PGs in the pathophysiology of HF by measuring plasma levels of metabolites of PGI2 and PGE2 in 15 patients with HF. Their results showed that levels of these PGs were three to 10 times higher than in normal subjects, and that they correlated with PRA and plasma AII levels. Finally, administration of an inhibitor of PG synthesis had harmful hemodynamic effects, particularly in patients with more severe HF and hyponatremia.56
PGs are produced in the kidneys, mainly in the medulla. They modulate responses to vasoconstrictor molecules and to certain vasodilators and release renin after stimulation of renal baroreceptors or the macula densa.57,58 The effects of some diuretics and of many vasodilators commonly used to treat cardiovascular diseases are in fact due to increased synthesis of natriuretic and vasodilator PGs.8,56–60 On the other hand, drugs that interfere with PG synthesis, such as many non-steroidal anti-inflammatory drugs, have a deleterious effect in HF.8,58,61 Production of NO, another endogenous vasodilator and natriuretic system, is closely connected to the relationship between PGs and the RAAS: NO and PGs physiologically counteract the renal effects of the RAAS, both hemodynamically and in sodium/water retention.62 Since renal PGs and NO both contribute to natriuresis and protect the afferent arteriole from the vasoconstrictor effects of AII, inhibition of the synthesis of either will accentuate the antrinatriuretic effects of the hormone.
Dopaminergic systemAs with the other neurohormonal systems, research into the dopaminergic system in the context of HF was triggered by the observation that the system was overstimulated, with elevated endogenous dopamine levels, in patients with the syndrome (Figure 2).63,64 It is now known that dopamine, as well as being an important cerebral neurotransmitter, is also a peripheral regulator with a significant role in renal and cardiovascular systems.65,66 Renal dopamine, most of which is synthesized in the kidneys themselves, plays a crucial part in modulating blood pressure, electrolyte balance, and renal and adrenergic function.65,66 Characterization of the dopaminergic receptors, which are divided into two groups – the D1 family and the D2 family – has shed further light on the role of dopamine in HF.8,67 The D1 receptors are localized post-synaptically, in effector organs such as the kidneys, coronary arteries, mesentery and brain, while the D2 receptors have a pre-synaptic location in the sympathetic terminals.8,65–67 Regarding their function, D1 receptor agonists cause hypotension, reduce afterload, increase blood flow to certain organs and increase sodium and water excretion, while D2 receptor agonists cause hypotension and bradycardia, reduce afterload and promote vasodilation in specific sites.8,65–67
Natriuretic peptide systemOf the neurohormonal systems involved in HF, the NPS is the one most recently to have been discovered and to have its importance recognized. In 1978, de Bold suggested that the granules found in atrial tissue in the mammalian heart, that had been first observed by Kisch 22 years before, were in fact peptides (Figure 2).40,68 In 1981 de Bold also reported a massive and rapid increase in water and sodium excretion when homogenates of these granules were injected into rats.69 Five years later, Burnett and colleagues detected a marked rise in plasma levels of atrial natriuretic peptide (ANP), the active substance in the granules identified by Kisch, in patients with HF (Figure 2),70 and in 1988, Sudoh and colleagues identified a new peptide isolated from pig brain with similar properties to ANP, which became known as brain-type natriuretic peptide (BNP) (Figure 2).71,72 It is now known that BNP is also produced in ventricular tissue. Finally, in the early 1990s, Sudoh and colleagues identified the third (and to date the last) peptide in the NPS, C-type natriuretic peptide (CNP).73 Several publications by Prof. Cerqueira Gomes and his team contributed to our understanding of the pathophysiological importance of these peptides.74–79
It is widely accepted that the NPS plays a key role in HF, counteracting the effects of overstimulation of the SNS, RAAS and vasopressin-arginine.40 Of the three known natriuretic peptides (NPs), ANP and BNP appear to be the most important for hormonal regulation of the heart. Synthesized mostly in the atria and ventricles, respectively, ANP and BNP are released in response to increased pressure and distension of cardiomyocytes.80,81 Of the three receptor types that bind to these peptides (NPR-A, NPR-B and NPR-C), the NPR-A receptor is responsible for most of the known physiological responses of the NPS.40,81 In terms of function, the NPs act on the kidney (causing vasodilation of the afferent arteriole and hence increasing glomerular filtration rate, as well as reducing water and sodium reabsorption), on blood vessels (stimulating vasodilation and reducing pre- and afterload), and on the heart (reversing the remodeling seen in HF via local regulation of collagen synthesis and cell hypertrophy, and inducing myocardial relaxation).40,80,81 In addition, they inhibit the production and secretion of aldosterone and renin, promote hemoconcentration, and have antifibrotic and antiapoptotic effects. It should be noted that, unlike other diuretics, the natriuretic effect of the NPS is not associated with potassium depletion.
In the early stages of HF, the effects of the NPs described above have a beneficial effect in maintaining physiological hemodynamics. However, as cardiac function continues to deteriorate, the NPS loses its effectiveness by one or more of the following means: decreased availability of the NPs, due to reduced production, increased removal or enzymatic degradation, particularly by neprilysin; decreased responsiveness to the NPs, due to reduced expression or desensitization of their receptors or inhibition of downstream signaling pathways; and the stronger effects of the neurohormonal systems that act against the NPS, i.e. the RAAS and SNS.80,81 Even so, plasma levels of the NPs remain high, and therefore measurement of plasma BNP is an indicator of disease severity and of prognosis.82
Neurohormonal antagonists in the treatment of heart failureThe rapid increase in knowledge of the neurohormonal systems in the early 1980s opened up new therapeutic possibilities centered on re-establishing the balance between the forces involved (Figure 2). The current approach is based on neurohormonal antagonists (Table 1), whose main characteristics, function and indications are summarized below. All of these therapies, both alone and combined with other drugs, have been evaluated at various stages of the disease, and have been shown to be associated with reductions in mortality and morbidity, including hospitalizations, in patients with HFrEF.
Neurohormonal antagonists currently used in the treatment of heart failure.
| Class | Examples | Therapeutic recommendationsa |
|---|---|---|
| ACEIs | Enalapril | First-line treatment, recommended for all symptomatic patients unless contraindicated or not tolerated. |
| Lisinopril | ||
| Ramipril | ||
| Trandolapril | ||
| Captopril | ||
| ARBs | Losartan | Recommended in patients who do not tolerate ACEIs. |
| Valsartan | ||
| Candesartan | ||
| BBs | Bisoprolol | Complementary to ACEIs: they can be started together as soon as HFrEF is diagnosed. Recommended in patients with a history of myocardial infarction and asymptomatic LV systolic dysfunction, angina or arrhythmias, and in asymptomatic patients with LV systolic dysfunction. |
| Carvedilol | ||
| Metoprolol succinate | ||
| Nebivolol | ||
| MRAs | Spirolactone | Recommended in patients who are still symptomatic after treatment with ACEIs and BBs, and those with HFrEF and LVEF≤35%. |
| Eplerenone | ||
| ARNI | Sacubitril/valsartan | Recommended as a replacement for ACEIs in ambulatory patients with HFrEF who remain symptomatic despite optimal treatment and who fulfill the inclusion criteria of the PARADIGM-HF trial. |
ACE: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; BBs: beta-blockers; LV: left ventricular; LVEF: left ventricular ejection fraction; MRAs: mineralocorticoid receptor antagonists.
ACEIs reduce the quantity of available AII by inhibiting its synthesis from angiotensin I, and increase the available quantity of bradykinin. They are currently the first-line treatment for various cardiovascular and renal diseases83 and are recommended in all patients with HFrEF except when contraindicated or not tolerated (Table 1). Several clinical trials conducted in recent years have demonstrated that ACEIs reduce mortality and morbidity in patients with HFrEF, including reducing rates of hospitalization, myocardial infarction and ventricular remodeling.1,83,84
Angiotensin receptor blockersARBs act downstream from ACEIs, by inhibiting AII signal transduction when its AT1 receptors are activated. Clinical trials on this drug class indicate that they are not superior to ACEIs in reducing mortality and morbidity associated with HFrEF, and they are therefore recommended only for patients who do not tolerate ACEIs (Table 1).1,83,84 Unlike ACEIs, ARBs hardly inhibit the degradation of bradykinin, a vasodilator enzyme that raises concentrations of PGs and NO and increases vascular permeability.40,83 Compared to ACEIs, secondary effects such as cough and angioedema are less common with ARBs.83,84
Beta-blockersBeta-blockers inhibit the SNS by blocking the signal transduction of norepinephrine and epinephrine via their receptors. When added to an ACEI or ARB, beta-blockers help reduce mortality and morbidity in patients with HFrEF.1,84 They can be classified according to their selectivity, as first-generation, non-selective (i.e. blocking both beta-1 and beta-2 receptors); second-generation, with greater affinity for beta-1 than for beta-2 receptors; and third-generation, which include both selective and non-selective agents, some with additional vasodilator effects.30 Not all beta-blockers are effective in the treatment of HF, but bisoprolol and metoprolol succinate (second generation), carvedilol (third generation, with a vasodilator effect due to additional alpha-adrenergic blockade), and nebivolol (with additional vasodilator effects dependent on NO release), have shown good results in clinical trials and have been approved for HF treatment in almost all cases.30
Mineralocorticoid receptor antagonistsMRAs inhibit the RAAS by blocking aldosterone signal transduction. They are recommended for patients medicated with an ACEI and a beta-blocker who are still symptomatic and who have left ventricular ejection fraction <35% (Table 1). According to the ESC guidelines, these drugs (spironolactone and eplerenone) are associated with lower cardiovascular mortality, but should be used with caution in patients with impaired renal function and plasma potassium >5.0 mmol/l.1,83
Renin inhibitorsDirect renin inhibitors block the RAAS at its first step, i.e. the conversion of angiotensinogen to angiotensin I. The first studies on renin inhibitors took place in the 1980s, but since their action is weak and non-specific, their bioavailability is low and their half-life is short, they were not recommended for HF treatment. However, trials on these drugs are under way and new renin inhibitors are being developed. It should also be noted that the main studies on the first available oral renin inhibitor, aliskiren, mainly concerned hypertension and diabetic nephropathy rather than HF. In the ATMOSPHERE trial in patients with chronic HF, the addition of aliskiren to enalapril led to more adverse events, and non-inferiority was not shown for aliskiren as compared with enalapril.85
Neprilysin inhibitorsNeprilysin is a key enzyme in the NPS, since it is responsible for the enzymatic degradation of ANP, BNP and CNP. The rationale for developing neprilysin inhibitors (NEPIs) is thus to increase the concentrations of these vasodilator peptides. However, neprilysin is not specific to NPs, but degrades a large number of other vasodilator peptides. Sacubitril is a NEPI developed in the 1990s that showed promising results in animal studies, but had varying and modest benefits in humans with HF and hypertension.40 It was found that sacubitril increased concentrations not only of vasodilator substances but also of vasoconstrictor hormones, particularly AII and endothelin I, and the resulting opposing forces tended to cancel each other out.40 Accordingly, NEPIs in isolation are not currently recommended for the treatment of HF.83
Vasopressin receptor inhibitorsSeveral vasopressin receptor inhibitors have been developed and have been tested in clinical trials, notably conivaptan (which inhibits the V1a and V2 receptors) and lixivaptan and tolvaptan (which inhibit the V2 receptor and are used in acute HF).48 The results are promising, showing improvements in dyspnea and hyponatremia, but there is no evidence of benefit for cardiovascular morbidity and mortality.
Endothelin receptor inhibitorsThe endothelin isoform ET-1 and its receptors ETA and ETB are synthesized and secreted by cardiomyocytes and other cardiac cells.86 Elevated ET-1 levels are associated with endothelial dysfunction, arterial stiffness, vasoconstriction, pulmonary hypertension and myocardial fibrosis.87 Although circulating ET levels are elevated in HF and are predictors of mortality, no beneficial effect on HF prognosis has been demonstrated by inhibition of ET receptors.55,88
Other innovative treatmentsGene therapy targeting the molecular mechanisms involved in HF has recently been seen as a potentially viable alternative treatment for the syndrome. Studies in this area have focused on mechanisms affecting myocardial contractility (particularly the beta-adrenergic system, proteins involved in calcium circulation and myofilaments), angiogenesis, cell protection and recruitment of stem cells. Stromal cell-derived factor 1-alpha (SDF-1) appears to play a central role in the latter mechanism, inducing the migration of bone marrow stem cells to damaged myocardium, promoting its regeneration.89 Preclinical studies have demonstrated that overexpression of SDF-1 promotes angiogenesis, improves cardiac function and reduces pathogenic cardiac remodeling in rats with ischemic HF.90
Another promising approach is that of sodium-glucose transporter 2 (SGLT2) inhibitors. These were first developed to improve glycemic control in diabetic patients, but two recent trials on two of these inhibitors (empagliflozin and canagliflozin) showed that patients with type 2 diabetes taking these drugs had relative risk reductions of 14% in cardiovascular death, non-fatal myocardial infarction and non-fatal stroke, 35% (empagliflozin) and 33% (canagliflozin) in HF hospitalizations, and 32% (empagliflozin) and 13% (canagliflozin) in all-cause death.91,92 The potential for SGLT2 inhibitors as HF therapy in patients both with and without diabetes, while as yet unproven, is currently being studied in clinical trials.93
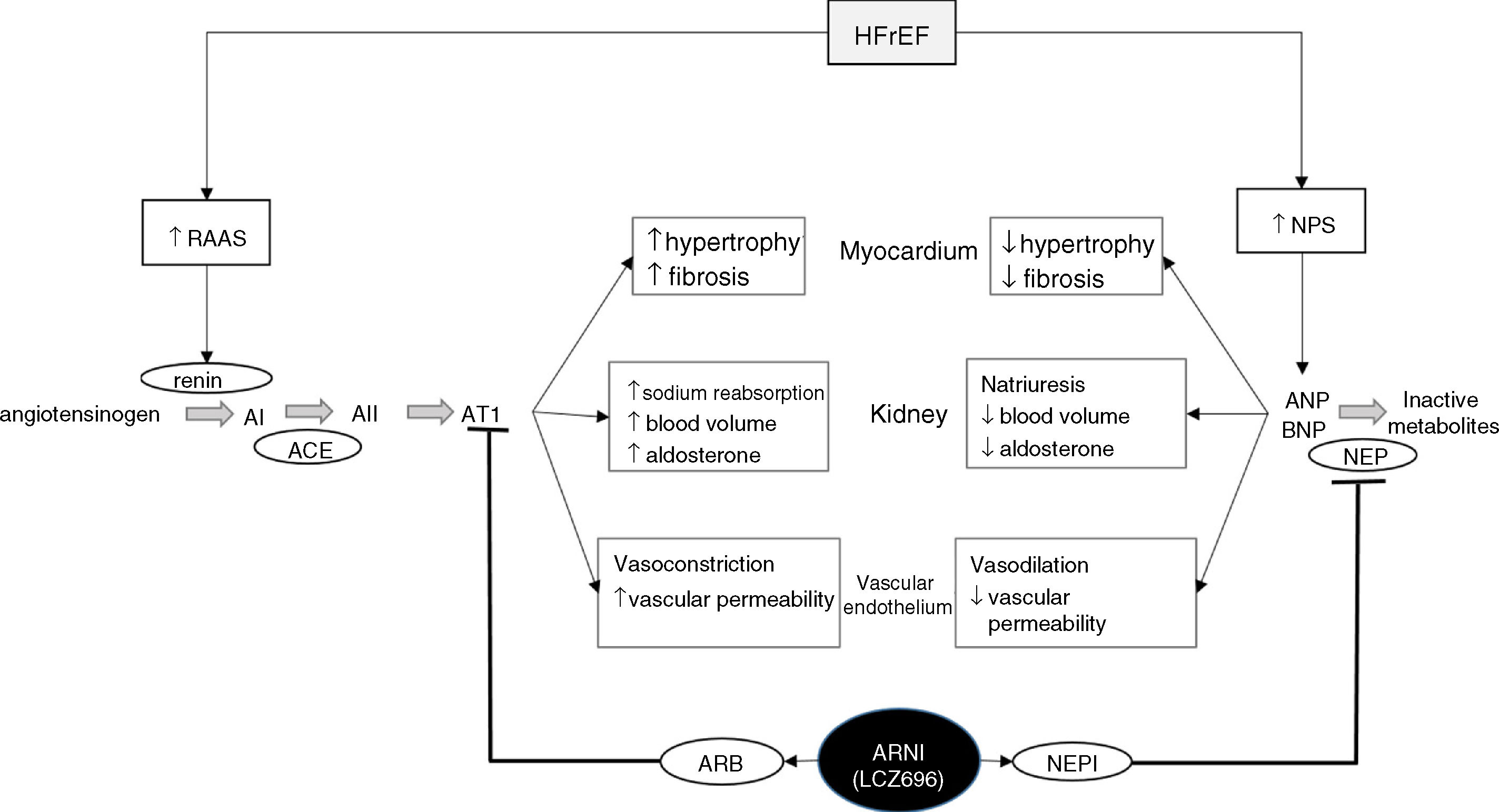
Sacubitril/valsartan – a new therapy from two old approachesThe latest drug approved for the treatment of HFrEF follows the modern trend for modulating neurohormonal pathways to achieve therapeutic objectives. Its innovative nature comes not from the molecules of which it is composed – sacubitril and valsartan, a NEPI and an ARB that had already been individually tested and, in the case of valsartan, marketed – but rather from their incorporation into a single drug (Figure 3). This HF therapy is designed not only to weaken potentially harmful neurohormonal mechanisms but also to strengthen cardiovascular protection mechanisms.
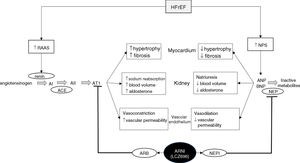
Diagram of the functioning of the renin-angiotensin-aldosterone and natriuretic peptide systems in heart failure, together with the action of sacubitril/valsartan, the first angiotensin receptor neprilysin inhibitor to be marketed. ACE: angiotensin-converting enzyme; AI: angiotensin I; AII: angiotensin II; ANP: atrial natriuretic peptide; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; AT1: angiotensin receptor 1; BNP: brain-type natriuretic peptide; HFrEF: heart failure with reduced ejection fraction; NPS: natriuretic peptide system; NEP: neprilysin; NEPI: neprilysin inhibitor; RAAS: renin-angiotensin-aldosterone system. Adapted from Ansara et al.99.
The main problem identified by tests on sacubitril was that it elevated AII levels, the vasoconstrictor effects of which counteracted the positive effects of increased NP levels.40 The obvious solution was to simultaneously inhibit AII. The first attempts were made in 1991 by Seymour and colleagues,40,94 who showed that adding an ACEI (captopril) to a selective NEPI in hypertensive rats resulted in greater blood pressure reductions than from either of the inhibitors given alone; these results were subsequently corroborated in other animal models.40,94
The logical conclusion was to try combining the two molecules in a single medication. Omapatrilat was the first and most thoroughly studied of the new drug class, known as vasopeptidase inhibitors.95 It initially sparked considerable enthusiasm; a comparison with the ACEI lisinopril gave promising results in terms of efficacy and safety.96 Similarly, in comparisons with enalapril, omapatrilat had a stronger antihypertensive effect, significantly reducing cardiovascular hospitalizations and mortality, and was not inferior to the ACEI in preventing HF hospitalization and all-cause death.97,98 However, the latter two trials revealed that omapatrilat was also associated with a greater incidence of angioedema, particularly in Afro-Americans. This higher angioedema rate is thought to be due to the adverse combination of the NEPI and the ACEI in blocking the breakdown of bradykinin.99 Given its effect on vasodilation and vascular permeability, increased bradykinin levels are probably the cause of these angioedemas.
The next step was the logical consequence of this observation: since ARBs, unlike ACEIs, do not block the breakdown of bradykinin, substituting an ARB promised to retain the beneficial effects of omapatrilat while avoiding its potential to cause angioedema. The result was the first angiotensin receptor neprilysin inhibitor (ARNI), sacubitril/valsartan (LCZ696), the function of which is outlined in Figure 3.
Efficacy, safety and recommendations for use of sacubitril/valsartanThe landmark trial demonstrating the therapeutic potential of sacubitril/valsartan was PARADIGM-HF by McMurray et al., the results of which were published in 2014.100 The trial was ended early, after a median follow-up of 27 months, due to clear evidence of the superiority of sacubitril/valsartan. The study design included 8442 symptomatic patients with HFrEF and aimed to compare sacubitril/valsartan with an ACEI (enalapril). Its results demonstrated that sacubitril/valsartan was superior in reducing cardiovascular and all-cause mortality and hospitalizations.100 In terms of safety, sacubitril/valsartan was associated with symptomatic hypotension, but there was no significant association with angioedema; although there was a non-significant tendency for greater occurrence of angioedema in the test group, when it did occur there was no upper airway compromise.100 Another analysis of the PARADIGM-HF data showed that sacubitril/valsartan not only reduced mortality but also had a beneficial effect on morbidity in patients with HFrEF, reflected in fewer patients requiring intensification of medical treatment for heart failure or an emergency department visit for worsening heart failure, fewer hospitalizations for worsening heart failure, and less need for intensive care, intravenous positive inotropic agents or implantation of a heart failure device or cardiac transplantation.101
These findings, taken together, led the ESC to recognize the therapeutic potential of sacubitril/valsartan. While not ignoring the need for long-term safety studies (considering, among other factors, the highly restrictive inclusion and exclusion criteria of PARADIGM-HF and the relatively small number of African-American patients included), the ESC guidelines recommend the use of sacubitril/valsartan instead of ACEIs in ambulatory patients with HFrEF who remain symptomatic despite optimal treatment and who fulfill the PARADIGM-HF criteria (Table 1).1
Finally, it is important to note that, although sacubitril/valsartan is only approved and recommended for the treatment of HFrEF, Solomon et al. showed that this drug also has potential for the treatment of HF with preserved ejection fraction (HFpEF) (ejection fraction ≥45%).102 In a phase II clinical trial, these investigators showed that reduction in N-terminal pro-BNP, a marker of left ventricular wall stress, at 12 weeks was greater in patients with HFpEF treated with sacubitril/valsartan than in patients receiving valsartan alone. Furthermore, patients in the sacubitril/valsartan group presented improved NYHA functional class at 36 weeks, as well as left atrial reverse remodeling.99,102 On the basis of these promising results, the PARAGON-HF (ClinicalTrials.gov identifier NCT01920711) is currently under way to assess the effects of sacubitril/valsartan on mortality and morbidity in patients with HFpEF, with a completion date in June 2019.
ConclusionHF is a complex clinical syndrome that progresses through various mechanisms and systems. Awareness of the impact of neurohormonal systems in the disease dates back to the 1980s, since when it has been progressively explored in various ways. Current HF therapy is based on modulating these neurohormonal systems, an approach that has proved beneficial in decreasing mortality and morbidity, including reducing hospitalizations. The latest drug approved for this purpose, sacubitril/valsartan, is unusual in acting on two of these systems simultaneously, weakening aggressive mechanisms and strengthening protective mechanisms. New therapeutic combinations can be expected in the future that will prolong survival and improve quality of life in patients with HF.
Conflicts of interestJorge Polónia has provided consulting services for Novartis, AstraZeneca Pharmaceuticals and Servier (companies that develop and market treatments for heart failure), has taken part in steering committees of clinical trials sponsored by Novartis and Sanofi/BMS, and has received speaker fees in sessions on heart failure and research grants from Novartis.
Francisco Rocha Gonçalves has no conflicts of interest to declare.
Funding for scientific editorial assistance was provided by Novartis Farma - Produtos Farmacêuticos S.A. We thank Catarina L. Santos, PhD (W4Research, Lda.) for her scientific editorial assistance with this manuscript.
MD, PhD. Faculty of Medicine Oporto, Portugal. Internal Medicine, Hypertension, Clinical Pharmacology. mailto:jjpolonia@gmail.com Professor of Internal Medicine of Faculty of Medicine of Porto, Portugal Full Professor (invited) of Medicine of University of Aveiro, Portugal Coordinator of Porto?s Pharmacovigilance Unit of Infarmed Senior Consultant of Hypertension, HTA and Cardiovascular Risk Unit, Hospital Pedro Hispano, Portugal Specialist in Internal Medicine & Clinical Pharmacology European Specialist in Clinical Hypertension (ESH) Past President of the Hypertension Association of Portuguese Society of Cardiology.
Please cite this article as: Polónia J, Gonçalves FR. A evolução histórica do envolvimento dos sistemas neuro-humorais no conhecimento da fisiopatologia e do tratamento da insuficiência cardíaca. Rev Port Cardiol. 2020;38:883–895.