We aimed to compare access to new health technologies to treat coronary heart disease (CHD) in the health systems of Portugal and the US, characterizing the needs of the populations and the resources available.
MethodsWe reviewed data for 2000 and 2010 on epidemiologic profiles of CHD and on health care available to patients. Thirty health technologies (16 medical devices and 14 drugs) introduced during the period 1980-2015 were identified by interventional cardiologists. Approval and marketing dates were compared between countries.
ResultsRelative to the US, Portugal has lower risk profiles and less than half the hospitalizations per capita, but fewer centers per capita provide catheterization and cardiothoracic surgery services. More than 70% of drugs were available sooner in the US, whereas 12 out of 16 medical devices were approved earlier in Portugal. Nevertheless, at least five of these devices were adopted first or diffused faster in the US. Mortality due to CHD and myocardial infarction (MI) was lower in Portugal (CHD: 72.8 vs. 168 and MI: 48.7 vs. 54.1 in Portugal and the US, respectively; age- and gender-adjusted deaths per 100000 population, 2010); but only CHD deaths exhibited a statistically significant difference between the countries.
ConclusionsDifferences in regulatory mechanisms and price regulations have a significant impact on the types of health technologies available in the two countries. However, other factors may influence their adoption and diffusion, and this appears to have a greater impact on mortality, due to acute conditions.
O objetivo deste estudo é comparar o acesso a novas tecnologias em saúde no tratamento da doença coronária (CHD), entre os sistemas de saúde de Portugal e dos Estados Unidos (US), caracterizando as necessidades das populações e disponibilidade de recursos.
MétodosForam comparados dados (2000 e 2010) de Portugal e US para descrever perfis epidemiológicos e recursos disponíveis na prestação cuidados de saúde na CHD. Trinta tecnologias de saúde (16 dispositivos médicos e 14 medicamentos), introduzidas durante 1980-2015, foram identificadas por cardiologistas de intervenção e calcularam-se as diferenças entre as datas de autorização de introdução no mercado/comercialização nos dois países.
ResultadosRelativamente aos US, Portugal apresenta perfis de risco mais baixos, menos hospitalizações per capita, menor número de centros per capita com valência para cateterismo coronário e cirurgia cardiotorácica. Mais de 70% dos medicamentos foram comercializados mais cedo nos US, enquanto 12 dos 16 dispositivos médicos obtiveram autorização para comercialização mais cedo em Portugal. Contudo, pelo menos cinco destes dispositivos foram adotados primeiro ou sofreram uma difusão mais rápida nos US. A mortalidade por CHD e enfarte agudo do miocárdio (EAM) foi inferior em Portugal (CHD: 72,8 [Portugal] versus 168 [US]; AMI: 48,7 [Portugal] versus 54,1 [US]; mortes por 100000 habitantes, padronizada por idade e sexo, 2010), tendo-se apenas verificado uma diferença significativa entre os países na mortalidade por CHD.
ConclusõesDiferenças nos mecanismos de regulação e controlo de preços têm um impacto significativo no tipo de tecnologias disponíveis nos dois países. Contudo, outros fatores influenciam a sua adoção e difusão, tendo um maior impacto na mortalidade em condições mais agudas.
American Heart Association
Conformité Européenne
coronary heart disease
cardiovascular disease
drug-eluting stent
European Union
Food and Drug Administration
intracoronary brachytherapy
Portuguese National Authority for Medicines and Health Products
in-stent restenosis
left ventricular support
myocardial infarction
National Health and Nutrition Examination Survey
percutaneous coronary intervention
percutaneous transluminal coronary angioplasty
transcatheter aortic valve replacement
The use of health technologies has grown dramatically in recent decades in developed countries, and now accounts for a considerable share of national health expenditure.1–7 Sustainability of access to health technologies has become a major priority in Portugal and worldwide, one that is grounded on ethical principles aiming at maximizing health gains given limited available resources.8 A national system for health technology assessment (SiNATS) is currently being implemented in Portugal. It is expected to extend the existing health technology assessment system to medical devices and to include new ways to support decision-making based on risk-sharing tools and real-world data monitoring.9 This context makes it an opportune time to assess contemporary access to medical devices and drugs in Portugal.
Different healthcare systems adopt new health technologies at different speeds and usage rates, leading to disparities in quality of care between patients in different countries.10–12 This results from a combination of various factors related to different barriers and needs, including the efficiency of the regulatory process, limitations of the reimbursement system, economic capacity, availability of resources, and the epidemiology of the target populations.11–14 Understanding these factors and how they relate to access to and initial use of health technologies is of crucial importance to improve quality of care, particularly when new health technologies have been proven to be cost-effective compared to previously available alternatives. International comparisons can provide insight into these issues by analyzing alternative settings, needs, and outcomes, and providing useful information on factors that are likely to affect access to health technologies. This exchange of experiences is crucial to generating enhanced information that can support decision-making for improved quality of care.
Treatment of coronary heart disease (CHD) has benefited significantly from technological innovations, both medical devices and drugs, which have improved clinical outcomes and quality of care.2,15–19 However, CHD remains one of the leading causes of death in most countries, with significant economic costs.2,20–23 With this in mind, our aim was to provide a contemporary historical overview of the situation in Portugal regarding access to innovations in health technology that are adjuvant or alternative CHD therapies against the backdrop of the risk profile of the population, available resources and mortality rates, using information from the US as a benchmark. The intention is to bridge the gap from previous international comparison studies assessing health technology access that typically focus on market-related aspects, few of which characterize populations’ needs and the resources available that may also impact access to and diffusion of new health technologies.11–13,24,25 We chose the US because of its contrasting healthcare system, which is driven by the private sector and is characterized by strikingly high per capita health expenditures, and because its regulatory agency, the Food and Drug Administration (FDA), is a world reference for drug and medical device assessment. Moreover, as reported by Danzon et al.,13 the US launched more drugs with shorter launch delays from approval compared to Portugal and other major markets during the 1990s. On the other hand, the efficiency of the European Union (EU)’s system for marketing approval of medical devices is often considered a key feature of the better access to medical devices in EU countries compared to the US.26–28 This makes the US an interesting contrast to Portugal that may improve our understanding of factors impacting access to and adoption of health technologies in the two health systems.
MethodsWe conducted a comprehensive literature review using publications from national governmental agencies, international organizations, professional associations, and scientific journals. Information was abstracted on prevalence of risk factors and diseases, hospitalization and mortality rates, infrastructure and human resources associated with CHD observed between 2000 and 2010, in Portugal and in the US. Approval dates of selected medical devices and drugs were abstracted for chronological characterization of access to healthcare technology using databases available at the FDA and the Portuguese National Authority for Medicines and Health Products (INFARMED) (personal contact with INFARMED).29–32
Epidemiologic profiles of coronary heart diseaseSelf-reported prevalence rates of risk factors for CHD in Portugal, including overweight and obesity, hypercholesterolemia, hypertension, diabetes and current smoking, were abstracted from the AMALIA study on the prevalence and distribution of cardiovascular risk factors in Portugal.33 The continuous datasets of the National Health and Nutrition Examination Survey (NHANES) were used to calculate comparable rates for the US, applying the same age inclusion criteria, time period and definitions of risk factors as in AMALIA whenever possible.33,34 Rates for the US were calculated for adults aged 40 years or older surveyed in the 2005-2006 and 2007-2008 NHANES cycles. Calculations of rates were based on data of self-perceived hypertension, hypercholesterolemia, and diabetes, defined as the participant's awareness of or current medication use for that condition, and smoking status was defined as one of the following: current smoker, ex-smoker or non-smoker. However, overweight/obesity prevalence rates for the US were based on actual measurements of weight and height, whereas AMALIA used self-reported measures. Age-adjusted prevalence rates were computed for comparison between the countries, standardized to the 2010 US population (Table A1 in Appendix A. Supplementary Material). US rates were computed using SAS 9.4 with code available on the NHANES tutorial website.35
CHD prevalence rates are not available for Portugal. Instead estimates of myocardial infarction (MI) prevalence were retrieved from the 2005-06 National Health Survey cycle and standardized to the 2010 US population controlling for gender and age.36 Both CHD and MI estimates for the US were abstracted from statistical reports available from the American Heart Association (AHA) for 2010.2 Crude per capita hospitalizations rates were produced based on the numbers reported by the AHA for the US, and by the Directorate-General of Health for Portugal divided by estimates of the total population.37–39
Crude rates of all-cause mortality and mortality from cardiovascular disease (CVD), CHD and MI were calculated from vital statistics data and estimates of the total populations.37,38,40,41 Age- and gender-adjusted mortality rates were obtained by direct standardization to the 2010 US population (Table A1 in Appendix A. Supplementary Material). Rate ratios were computed for analysis of within- and between-country variations in death rates.
Medical devices and drugsA list of procedures and medical device categories and another of active substances and therapeutic uses were drawn up by interventional cardiologists who gave their subjective assessment as to which were technological breakthroughs in the field of interventional cardiology between January 1980 and February 2015. Several cardiovascular devices reviewed were outside the scope of CHD treatment, but were included for a better understanding of the dynamics of the two healthcare systems. After this list was drawn up, the first device model approved for each procedure and device category in each of the two countries was then determined. Similarly, the first drug brand approved for marketing for each active substance and therapeutic use was identified.
In the US, approval dates of most medical devices and drugs are available from FDA databases.30–32 The only approval date that had to be retrieved from a medical journal was of the first percutaneous transluminal coronary angioplasty (PTCA) balloon. In Portugal, approval and marketing dates of drugs are well documented but medical device data are not. We therefore relied on a combination of different sources of information to obtain dates that devices obtained the CE (Conformité Européenne) mark. The CE mark is the certification attributed to certain products, including medical devices, when approved for marketing in the EU. These sources included medical device industry representatives, online press releases and news agencies, medical device industry associations in the EU and Portugal (Eucomed and the Portuguese Association of Medical Device Companies [APORMED]), and scientific journals. However, approval dates of some devices were not available for Portugal and the dates of first use were considered as a time reference of their approval. These dates refer to the first use in the interventional cardiology ward of Santa Cruz Hospital, which is a reference hospital and pioneer in many interventional cardiology procedures in Portugal.42 Both approval and marketing dates of drugs were abstracted from INFARMED, but only marketing dates were used for comparison with the US in the results.
First device models and drug brands were determined according to the earliest approval date among their device or active substance class in each country. For comparison between countries, the lag in access to healthcare technology between the US and Portugal was defined as the difference between approval/first use/marketing dates, calculated as the US date minus the date for Portugal. February 13, 2015, the most recent date for which data are available, was used to compute the difference if the technology was still unavailable in one of the countries. In the event of missing information on the specific day or month of dates, the last day of the month or the month of June, respectively, were used to calculate the difference.
Human and infrastructure resourcesIn order to characterize the available human and infrastructure resources, information was collected on the number of cardiothoracic surgeons, cardiologists, hospitals with cardiac catheterization laboratories and hospitals with cardiac surgery facilities in 2000 and in 2010 for the US and Portugal (personal contact with the hospitals).43–45 Per capita rates were then computed based on estimates of the total populations of the two countries.37,38
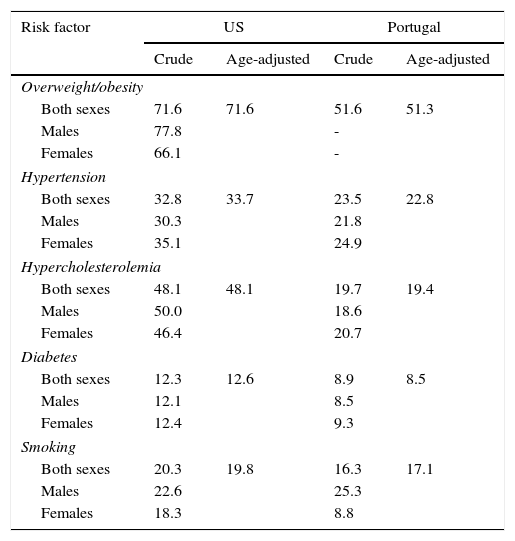
ResultsIn 2010, the Portuguese population was 10.6 million, 19% of whom were aged 65 years or more, and healthcare coverage was universal; the 2010 US population was 309.3 million, 13% of whom were aged 65 years or older, and 16.3% had no health insurance (Table A2 in Appendix A. Supplementary Material). Table 1 presents crude and age-adjusted prevalence rates of CHD risk factors, indicating lower numbers for Portugal compared to the US.
Self-reported prevalence rates (%) of risk factors in the US and Portugal in adults aged ≥40 years.
| Risk factor | US | Portugal | ||
|---|---|---|---|---|
| Crude | Age-adjusted | Crude | Age-adjusted | |
| Overweight/obesity | ||||
| Both sexes | 71.6 | 71.6 | 51.6 | 51.3 |
| Males | 77.8 | - | ||
| Females | 66.1 | - | ||
| Hypertension | ||||
| Both sexes | 32.8 | 33.7 | 23.5 | 22.8 |
| Males | 30.3 | 21.8 | ||
| Females | 35.1 | 24.9 | ||
| Hypercholesterolemia | ||||
| Both sexes | 48.1 | 48.1 | 19.7 | 19.4 |
| Males | 50.0 | 18.6 | ||
| Females | 46.4 | 20.7 | ||
| Diabetes | ||||
| Both sexes | 12.3 | 12.6 | 8.9 | 8.5 |
| Males | 12.1 | 8.5 | ||
| Females | 12.4 | 9.3 | ||
| Smoking | ||||
| Both sexes | 20.3 | 19.8 | 16.3 | 17.1 |
| Males | 22.6 | 25.3 | ||
| Females | 18.3 | 8.8 | ||
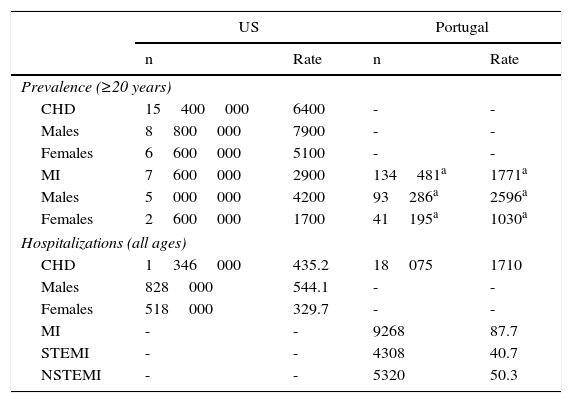
In 2010, 64 out of 1000 US adults had CHD, of whom approximately half had suffered an MI. We were unable to obtain CHD prevalence rates for Portugal, but MI was less frequent in Portugal than in the US in 2010 (US: 2900 vs. Portugal: 1687, age- and gender-adjusted rates per 100000 population) (Table 2). Furthermore, the number of hospitalizations per capita in the US was almost three times higher than in Portugal (US: 435.2 vs. Portugal: 171.0, crude rates per 100000 population) (Table 2), which is consistent with the populations risk profiles in Table 1. However, it should be noted that the figures for Portugal refer to hospitalizations in coronary care units, whereas US hospitalizations refer to any hospitalization for CHD at an acute care hospital.
Coronary heart disease prevalence rates (per 100000 population) in the US and Portugal, 2010.
| US | Portugal | |||
|---|---|---|---|---|
| n | Rate | n | Rate | |
| Prevalence (≥20 years) | ||||
| CHD | 15400000 | 6400 | - | - |
| Males | 8800000 | 7900 | - | - |
| Females | 6600000 | 5100 | - | - |
| MI | 7600000 | 2900 | 134481a | 1771a |
| Males | 5000000 | 4200 | 93286a | 2596a |
| Females | 2600000 | 1700 | 41195a | 1030a |
| Hospitalizations (all ages) | ||||
| CHD | 1346000 | 435.2 | 18075 | 1710 |
| Males | 828000 | 544.1 | - | - |
| Females | 518000 | 329.7 | - | - |
| MI | - | - | 9268 | 87.7 |
| STEMI | - | - | 4308 | 40.7 |
| NSTEMI | - | - | 5320 | 50.3 |
CHD: coronary heart disease; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction.
Self-reported prevalence of myocardial infarction in adults (≥25 years) abstracted from the National Health Survey, 2005/2006 cycle.36
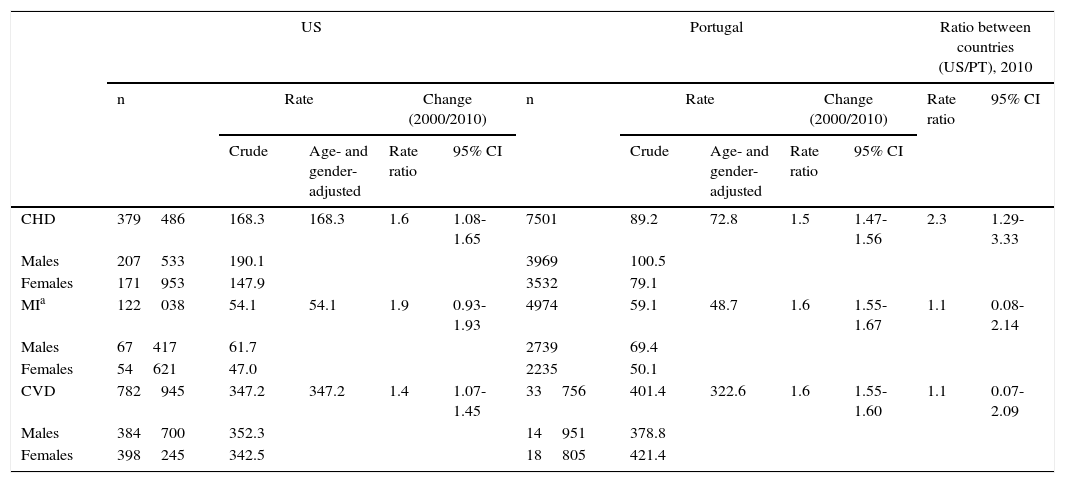
Age- and gender-adjusted death rate ratios between 2000 and 2010 show a significant decrease in mortality due to CVD and CHD in both countries, but only Portugal exhibited a statistically significant decrease in MI mortality in this decade. Relative to the US, CHD mortality rates were significantly lower in Portugal in 2010, but there was no statistically significant difference in CVD or MI mortality rate ratios between the two countries (Table 3).
Coronary heart disease mortality rates (per 100000 population) in the US and Portugal, 2010, age ≥20 years.
| US | Portugal | Ratio between countries (US/PT), 2010 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Rate | Change (2000/2010) | n | Rate | Change (2000/2010) | Rate ratio | 95% CI | |||||
| Crude | Age- and gender-adjusted | Rate ratio | 95% CI | Crude | Age- and gender-adjusted | Rate ratio | 95% CI | |||||
| CHD | 379486 | 168.3 | 168.3 | 1.6 | 1.08-1.65 | 7501 | 89.2 | 72.8 | 1.5 | 1.47-1.56 | 2.3 | 1.29-3.33 |
| Males | 207533 | 190.1 | 3969 | 100.5 | ||||||||
| Females | 171953 | 147.9 | 3532 | 79.1 | ||||||||
| MIa | 122038 | 54.1 | 54.1 | 1.9 | 0.93-1.93 | 4974 | 59.1 | 48.7 | 1.6 | 1.55-1.67 | 1.1 | 0.08-2.14 |
| Males | 67417 | 61.7 | 2739 | 69.4 | ||||||||
| Females | 54621 | 47.0 | 2235 | 50.1 | ||||||||
| CVD | 782945 | 347.2 | 347.2 | 1.4 | 1.07-1.45 | 33756 | 401.4 | 322.6 | 1.6 | 1.55-1.60 | 1.1 | 0.07-2.09 |
| Males | 384700 | 352.3 | 14951 | 378.8 | ||||||||
| Females | 398245 | 342.5 | 18805 | 421.4 | ||||||||
CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; MI: myocardial infarction; PT: Portugal.
Includes subsequent MI. Rates standardized to the 2010 US population (Table A1 in Appendix A. Supplementary Material).
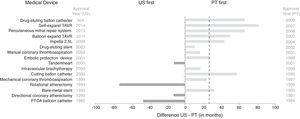
Figures 1 and 2 depict the lag in access to selected devices and drugs between the US and Portugal. Brand names and their approval/first use/marketing dates have been omitted from this article but are available as supplementary material (Tables A4 and A5 of Appendix A. Supplementary Material).
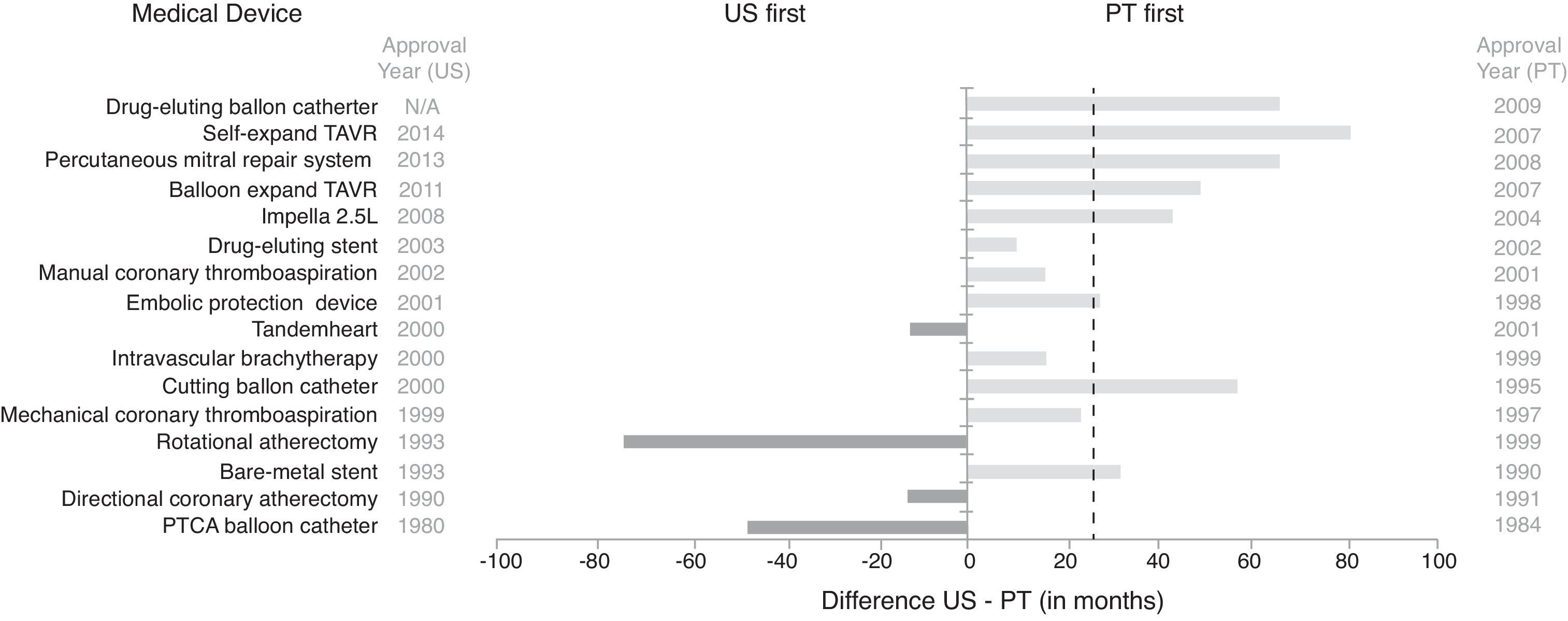
Lag in access to selected interventional cardiology devices between the US and Portugal. Dashed line represents median time lag (US-Portugal). N/A: not available; PT: Portugal; PTCA: percutaneous transluminal coronary angioplasty; TAVR: transcatheter aortic valve repair. The lag in access to devices was determined based on approval dates for the US available from the Food and Drug Administration (in one case from a medical journal), and, for Portugal, dates of CE marking retrieved from personal communication with medical device industry representatives, online press releases, EUCOMED, and scientific journals, and first use dates in Santa Cruz Hospital (raw data available in Table A4 in Appendix A. Supplementary Material).
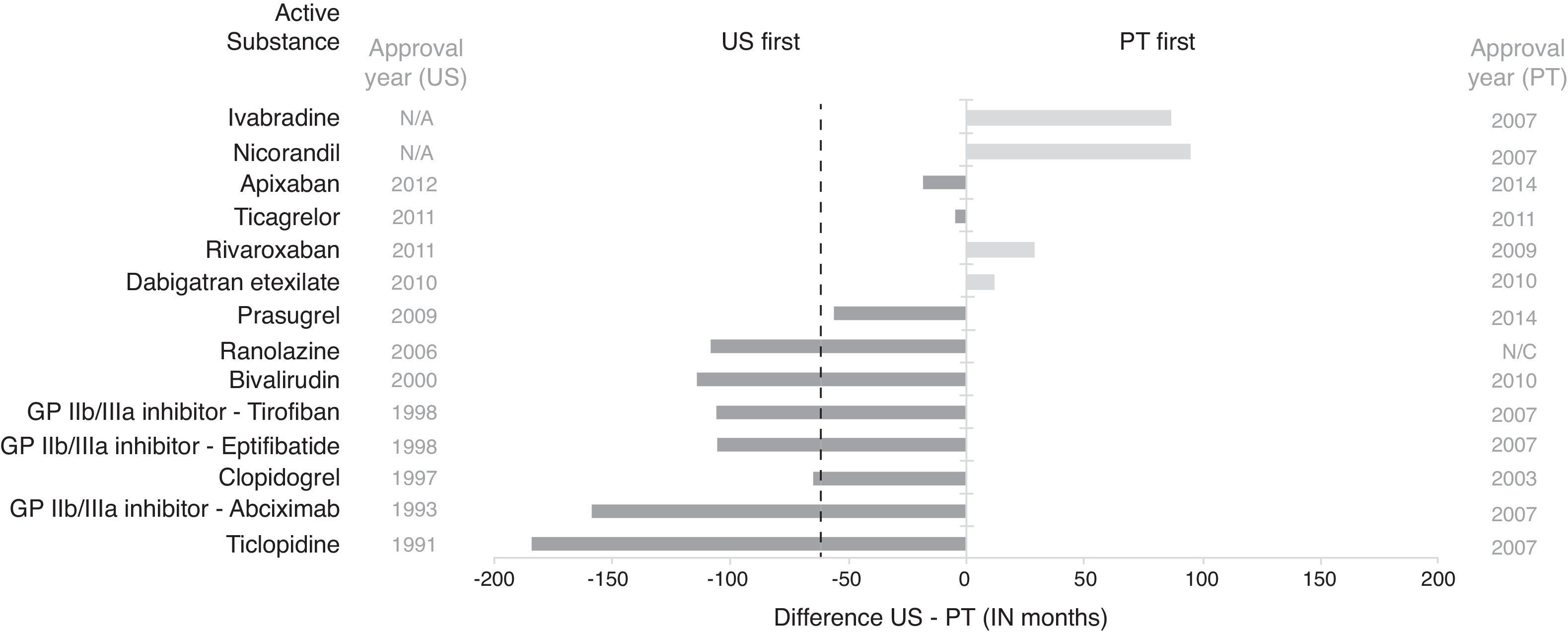
Lag in access to selected interventional cardiology drugs between the US and Portugal. Dashed line represents median time lag (US-Portugal). GP: glycoprotein; N/A: not available; N/C: not marketed; PT: Portugal. Lag in access to active substances was determined based on approval dates available for the US from the Food and Drug Administration and marketing dates for Portugal from INFARMED (raw data available in Table A5 in Appendix A. Supplementary Material).
Access to medical devices in the US is slower than in Portugal, with only four of the 16 devices reviewed being available first in the US. Moreover, on average, it took 22 months longer to approve the first device model in the US than in Portugal (median time difference 27 months). The drug-eluting balloon for percutaneous coronary intervention (PCI) was the most recent device approved in Portugal, in 2009, and this is still unavailable in the US.
New drugs are more readily available in the US than in Portugal. Only four out of the 14 drugs included in this study were available first in Portugal. On average, drugs were available 51 months sooner in the US (median time difference 61 months). However, up to February 13, 2015, nicorandil and ivabradine had not been approved in the US but have been marketed in Portugal since 2007, and ranolazine had not been marketed in Portugal, despite approval in Portugal in 2008 and in 2006 in the US.
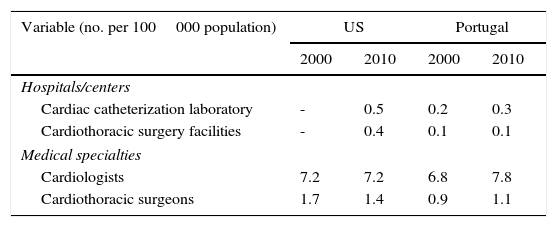
Table 4 provides information regarding diagnostic and treatment facilities as well as medical specialties available in each country. Relative to Portugal, in 2010 the US had nearly double the number of hospitals per capita with cardiac catheterization facilities and four times the number of hospitals per capita with cardiothoracic surgery services. However, while the number of cardiothoracic surgeons per capita was higher in the US, there were more cardiologists per capita in Portugal.
Availability of specialized health facilities and personnel for treatment of coronary heart disease in the US and Portugal, 2000 and 2010.
| Variable (no. per 100000 population) | US | Portugal | ||
|---|---|---|---|---|
| 2000 | 2010 | 2000 | 2010 | |
| Hospitals/centers | ||||
| Cardiac catheterization laboratory | - | 0.5 | 0.2 | 0.3 |
| Cardiothoracic surgery facilities | - | 0.4 | 0.1 | 0.1 |
| Medical specialties | ||||
| Cardiologists | 7.2 | 7.2 | 6.8 | 7.8 |
| Cardiothoracic surgeons | 1.7 | 1.4 | 0.9 | 1.1 |
The number of centers providing cardiothoracic surgery in the US in 2000 was not available at the Area Health Resource Files (AHRF).43 The number of centers providing cardiac catheterization in 2000 was omitted given that the AHRF used different measures over the years, but numbers for 2010 were consistent with those reported by the American Hospital Association Annual Survey Database for the fiscal year 2010.
There are dramatic differences between Portugal and the US regarding the availability of healthcare technologies and resources for the treatment of coronary heart disease, as well as different epidemiologic risk profiles.
Medical devicesOf the medical devices studied, the majority were approved earlier in the EU/Portugal than in the US, showing that the EU approval system is more time efficient than that of the US (the FDA), as reported by other researchers.10,26,28 This study adds to the current evidence that this time advantage remains when considering the first model of a new technological family.
Earlier approval does not always translate into earlier use of devices. The first medical device approved for intracoronary brachytherapy (ICB) received the CE mark in 1999 but only diffused into Portugal in January 2001. This was motivated by the FDA's approval of the Novoste Beta-Cath system (Novoste Corp.) in that year, establishing ICB as an adjuvant therapy of PCI for in-stent restenosis (ISR).46 Nevertheless, it ceased to be used within six months of its adoption. In the US, ICB saw moderate and decreasing use due to a combination of factors, including the approval of drug-eluting stents (DES) two years later, which significantly reduced the occurrence of ISR, the logistic complexity associated with ICB, and the increasing evidence favoring DES over ICB to treat ISR.19,47–49 Dangas et al. reported that most hospitals no longer possess the necessary facilities to provide ICB in the US.50
DES were rapidly adopted in both countries after their approval. Routine implantation of DES began immediately after approval, in 2002 in Portugal and in 2003 in the US.51,52 By 2003, Portugal had the highest rate of DES implantation in Europe, in 55% of PCI procedures, and by the third quarter of 2005 the use of DES per PCI peaked at nearly 90% in the US.51,53 However, it should be noted that Portugal has the lowest primary PCI rates per capita in Western Europe and, until 2007, thrombolysis was more commonly used than angioplasty in the treatment of ST-elevation myocardial infarction.54,55 The number of PCIs per in-patient case in Portugal is less than half of that in the US.5 This is also consistent with the established capacity to treat patients assessed by the number of specialized physicians and existing infrastructure in each country (Table 4). Considerable differences were observed between the two countries regarding the per capita number of cardiothoracic surgeons and specialized facilities. Only the number of cardiologists per capita was comparable. Moreover, the balance between the number of cardiac catheterization laboratories, cardiac surgery centers, interventional cardiologists and cardiothoracic surgeons, and the potential number of patients to treat is an important but complex topic of discussion with impact on access and quality of care.51,56,57 Although outside the scope of this paper, this is an important issue, given the public vs. private nature of the two healthcare systems, their economic capacity, and observed differences in CHD incidence (Tables 2 and A2).
To our knowledge there are few publications reporting on the real-world implantation of left ventricular support (LVS) devices, specifically the Impella 2.5 (Abiomed Inc.) and TandemHeart (CardiacAssist Inc.). Results from the Europella, EUROSHOCK and USpella registries indicate that the US was faster in adopting the Impella, perhaps in response to early European experience with these devices. Three years after the approval of the Impella (data collected between July 2004 and December 2007), 144 elective high-risk PCI patients with prophylactic LVS using the Impella were registered in the Europella registry over 10 European centers, including one from Portugal.58 Hence, on average, five patients per center per year were implanted with this device. Maini et al. reported the first results in the USpella registry of 276 high-risk PCI patients across 34 US centers.59 The authors do not clearly specify the reporting period of the data but given the initial collection date (March 2010) and the submission date of the article (June 2011), it can be assumed that these numbers represent a one-year span. Thus, on average, eight patients per center per year were implanted with the Impella 2.5. It is worth noting that the intra-aortic balloon remains the most frequently used device for LVS.60–62
Transcatheter aortic valve replacement (TAVR) devices also saw early adoption and rapid increase in use following market approval in both countries.63,64 According to the TAVR registry in Portugal, TAVR use plateaued in 2010 with more than 20 implants per center (6.1 implants per million population).64 In the US, Brennan et al. observed a significant increase in the volume of TAVR use based on TAVR records collected between 2008 and 2013.63 In 2012, one year after the first TAVR device was approved by the FDA, approximately 20 TAVR procedures per center were performed (15.8 implants per million population).
There was a five-year gap between approval of the MitraClip device (Abbott Vascular) in Portugal and the US. However, according to numbers presented at a scientific conference in April 2014, the first case treated using the MitraClip in Portugal was in late 2012, four years after its market approval.65 Since then, a total of nine cases have been treated with this device.65 In the US, adoption appears to have been much faster, given that more than five hundred cases had been treated in the US by the end of August 2014, around a year after its approval, according to figures from the TVT registry presented by Sorajja et al. at the American College of Cardiology 2015 Scientific Sessions.66 Furthermore, by August 2014, there were 76 centers in the US providing MitraClip therapy (around 0.25 centers per million population) as opposed to two in Portugal (0.19 centers per million population).67,68
DrugsIn contrast to devices, information on approval dates of drugs in Portugal is readily available. In addition to approval dates, INFARMED provides information on effective marketing dates of drugs. On average, marketing of drugs in Portugal was delayed by 81 months from approval (median 65 months lag). Although almost 60% of the selected drugs were approved earlier in Portugal, 61% were marketed earlier in the US (Figure 2 and Table A5 in Appendix A. Supplementary Material). In particular, outpatient prescription of clopidogrel in Portugal started in 2003, the same year this drug became eligible for reimbursement and the same year that effective marketing is reported by INFARMED.12 By contrast, in the US, sales of clopidogrel, marketed as Plavix, have increased since its approval in 1997.69 Longer launch times in Portugal may be driven by both implementation of maximum prices and reimbursement regulations that aim to control the governmental budget, but also depend on the launch strategies of pharmaceutical companies, which ultimately decide when to launch new drugs onto the market. Given the pricing rules of each country, there will be a specific order to be followed by each proposed drug, so as to optimize pricing. External (cross-country) price referencing means that low prices tend to spill over across countries, often delaying launches of new drugs by companies in countries with low expected prices and small volume sales such as Portugal.13
Implications of results on mortalityOur results show that CHD deaths were significantly lower in Portugal compared to the US, but no statistical difference was observed for MI deaths between countries. These observations, combined with the fact that MI is the most acute manifestation of CHD, for which the use of health technologies by trained professionals and adequately equipped health facilities may prolong both duration and quality of life, suggest that differences between Portugal and the US in available resources and access to health technologies (faster access to drugs, earlier adoption and/or faster diffusion of devices, and more specialized health facilities and professionals in the US compared to Portugal) may play an important role in quality of care, mitigating differences in epidemiologic risk profiles. Nevertheless, only Portugal exhibited a statistically significant decrease in MI mortality in the decade under study, suggesting that healthcare for these patients has significantly improved in Portugal. Therefore, it is possible that the trends of approval lags observed in Figures 1 and 2, increasingly favorable to Portugal in more recent years, may have contributed to this improvement.
LimitationsOur study has some limitations. First, rates based on self-perceived measurements likely underestimate the prevalence of risk factors. However, additional evidence confirms that most risk factors are less prevalent in Portugal than the US. Only hypertension appears to be more prevalent in Portugal (further details available as supplementary material). Furthermore, risk profiles were determined considering the overall populations of the two countries. The actual profiles of the CHD populations were not studied, which could explain the differences in usage patterns and mortality rates. Second, routine in-hospital use of clopidogrel, ticlopidine, and abciximab in Portugal began prior to the marketing date provided by INFARMED.53,70–72 Particularly in the case of the drugs indicated for exclusive hospital use (e.g. glycoprotein IIb/IIIa inhibitors), these reports substantially contradict INFARMED's marketing dates. Therefore, information on marketing should be regarded with caution with regard to drugs that are to be used in-hospital only. Third, the brief discussion on adoption and diffusion of healthcare technology is based on key scientific papers for a few technologies. A deeper understanding of the adoption mechanisms is thus required. Furthermore, death certificates may inaccurately state the cause of death and fail to describe the effectiveness of technologies. There is thus a need for better knowledge of the diffusion patterns and effectiveness of health technologies, ideally through the development and analysis of equivalent datasets from the two healthcare systems.
ConclusionsOur results show that differences in regulatory mechanisms and price regulations have a significant impact on market access strategies and on the types of treatment available for CHD, benefiting the US in the case of drug availability while favoring Portugal in the case of medical devices. Differences in risk profiles and available resources may partially explain the differences in adoption of devices after approval on the two sides of the Atlantic, as at least five devices considered were adopted first or diffused faster in the US despite the initial advantage of earlier approval in Portugal. Other factors may also play an important role. On the other hand, the ability of the US to adopt and diffuse health technologies faster and over more centers may have contributed to better quality of acute care compared to Portugal, mitigating differences between the two countries in epidemiologic risk profiles.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by the Harvard Medical School – Portugal Program (HMSP-ICT/0013/2011). Armando Teixeira-Pinto was partially supported by National Health and Medical Research Council program grant 633003 to the Screening and Test Evaluation Program (STEP).
Conflicts of interestThe work was conducted under a grant from the Harvard Medical School – Portugal Program. ATP was partially supported by an NHMRC Program Grant. SLN is under contract with the Massachusetts Department of Public Health to monitor the quality of cardiovascular procedures and surgeries in all non-federal acute care Massachusetts hospitals, and provides statistical consultation to the Yale New Haven Hospital for their contract with the US Centers for Medicare and Medicaid Services to develop and apply hospital-specific public reports involving cardiovascular disease as well as care provided in accountable care organizations, for which she receives fees.
No conditions were imposed by the grant providers in the development of the study or preparation of the manuscript.
The authors would like to thank the Northern Pharmacovigilance Centre, Portugal, for their contribution and insight regarding innovative drugs and approval mechanisms of drugs in Portugal, and the medical device industries and associations for sharing information on approval dates of devices, including CardiacAssist Inc., Boston Scientific, Vascular Solutions, Inc., B Braun Melsungen AG, Medtronic Portugal, UP Medical and Eucomed. We would also like to thank the Portuguese Society of Cardiology and heads of cardiology wards of Portuguese hospitals for providing information regarding the dates that cardiac catheterization laboratories began operating in their hospitals. Particularly, the authors would like to thank the interventional cardiology ward of Santa Cruz Hospital for sharing some pages of the commemorative book entitled 20 anos de Angioplastias Coronárias no Hospital de Santa Cruz (1984-2004), which contains dates of first use of most technological innovations reviewed in this work. Finally, the authors would like to thank INFARMED for sharing the dates of marketing of drugs in Portugal.


 PTCA: percutaneous transluminal coronary angioplasty;
PTCA: percutaneous transluminal coronary angioplasty;  INFARMED (raw data available in Table A5 in Appendix A. Supplementary Material).' title='Lag in access to selected interventional cardiology drugs between the US and Portugal. Dashed line represents median time lag (US-Portugal). GP: glycoprotein; N/A: not available; N/C: not marketed; PT: Portugal. Lag in access to active substances was determined based on approval dates available for the US from the Food and Drug Administration and marketing dates for Portugal from
INFARMED (raw data available in Table A5 in Appendix A. Supplementary Material).' title='Lag in access to selected interventional cardiology drugs between the US and Portugal. Dashed line represents median time lag (US-Portugal). GP: glycoprotein; N/A: not available; N/C: not marketed; PT: Portugal. Lag in access to active substances was determined based on approval dates available for the US from the Food and Drug Administration and marketing dates for Portugal from 

