We describe the case of a 76-year-old man with a history of ischemic heart disease and functional mitral regurgitation, who over the previous six months had experienced worsening of functional class (NYHA III/IV) under optimal medical therapy, without ischemic symptoms and with negative ischemic tests. Mitral valve annuloplasty was considered. As the patient presented left bundle branch block on the surface ECG, cardiac resynchronization therapy (CRT) was also considered. There was, however, severe biventricular dysfunction and moderate to severe pulmonary hypertension, which are considered predictors of non-response to CRT. On echocardiographic evaluation of mechanical dyssynchrony by two-dimensional strain (2DS), spectral Doppler and color tissue Doppler imaging (TDI)/tissue synchronization imaging (TSI), we observed absence of atrioventricular dyssynchrony and presence of interventricular dyssynchrony, with inconclusive intraventricular longitudinal dyssynchrony, but with marked intraventricular radial dyssynchrony.
The latter, immediately observed on the two-dimensional image, and termed multiphasic septal motion or septal flash, was characterized and quantified with 2DS. In our experience, the presence of such septal motion, for which the substrate is predominantly radial dyssynchrony, is a predictor of CRT response. Weighing the risks and benefits of mitral valve annuloplasty without associated revascularization versus CRT, we opted for the latter. Marked improvement in clinical and echocardiographic parameters was observed, compatible with the current criteria for “responder”. The improvement began one month after implantation and continued throughout 2-year follow-up. In this case, detailed echocardiographic study of mechanical synchrony enabled the most appropriate and effective therapeutic strategy to be chosen.
Descreve-se o caso clínico de um doente de 76 anos, com antecedentes de cardiopatia isquémica e regurgitação mitral grave funcional, que nos últimos 6 meses se encontrava em classe funcional NYHA III/IV, sob terapêutica médica optimizada, na ausência de sintomas isquémicos e com testes de isquémia negativos. Foi considerada a hipótese de cirúrgica de anuloplastia mitral de redução, mas como o doente apresentava no ECG de superfície padrão de bloqueio completo do ramo esquerdo (BCRE), optou-se por terapeutica de ressincronização (CRT), apesar de existir disfunção biventricular grave e hipertensão pulmonar (HTP) moderada a grave, factores considerados predictores de não-resposta a CRT. Na avaliação ecocardiográfica de dessincronia mecânica por Strain Bidimensional (2DS), Doppler espectral e Doppler tecidular cor sincronizado para a sistole (TDI/TSI) verificou-se a ausência de dessincronia aurículo-ventricular, a presença de dessincronia interventricular, sendo a análise da dessincronia intraventricular longitudinal ambígua, mas com evidente dessincronia intraventricular radial. Esta última era desde logo evidente na apreciação visual da imagem bidimensional e modo M, podendo ser descrita como movimento septal multifásico, ou septal flash, tendo sido caracterizada e quantificada por 2DS. Na nossa experiência, a presença deste movimento septal que tem como substrato dessincronia intraventricular de predomínio radial, parece ser um marcador de resposta a CRT, pelo que, ponderados os riscos/benefícios da terapêutica cirúrgica mitral sem revascularização associada, versus CRT, optou-se por esta última, tendo sido observado comportamento de resposta clínica e ecocardiográfica compatível com a actual designação de “respondedor”, registada ao primeiro mês e mantida no seguimento de dois anos. Neste caso o estudo ecocardiográfico detalhado do sincronismo mecânico, permitiu a escolha da estratégia terapêutica mais adequada e eficaz.
Cardiac resynchronization therapy (CRT) has been shown to improve quality of life and survival in a significant number of patients with heart failure (HF) in randomized multicenter clinical trials.1–3 The inclusion criteria of these trials – New York Heart Association (NYHA) class III/IV, QRS ≥120ms and ejection fraction (EF) ≤35% – were subsequently incorporated into current guidelines.4 Many non-randomized single-center studies in three continents have shown additional benefit when there is evidence of mechanical dyssynchrony on imaging studies.5–7 We report the case of a patient with ischemic dilated cardiomyopathy who met the criteria for CRT but had severe functional mitral regurgitation, moderate to severe pulmonary hypertension (PH) and associated right ventricular dysfunction, in whom a biventricular pacemaker with cardioverter-defibrillator (CRT-ICD) was implanted.
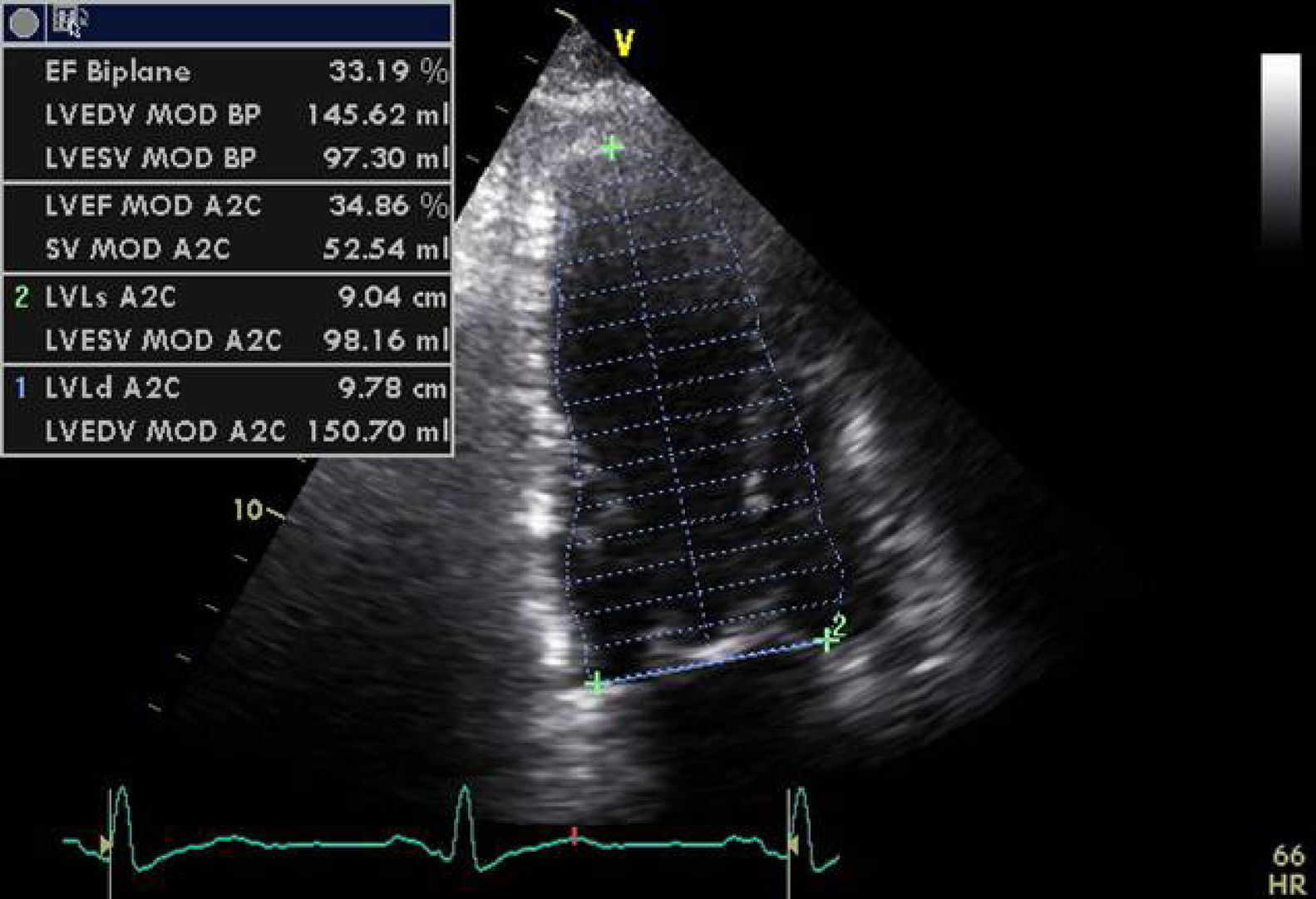
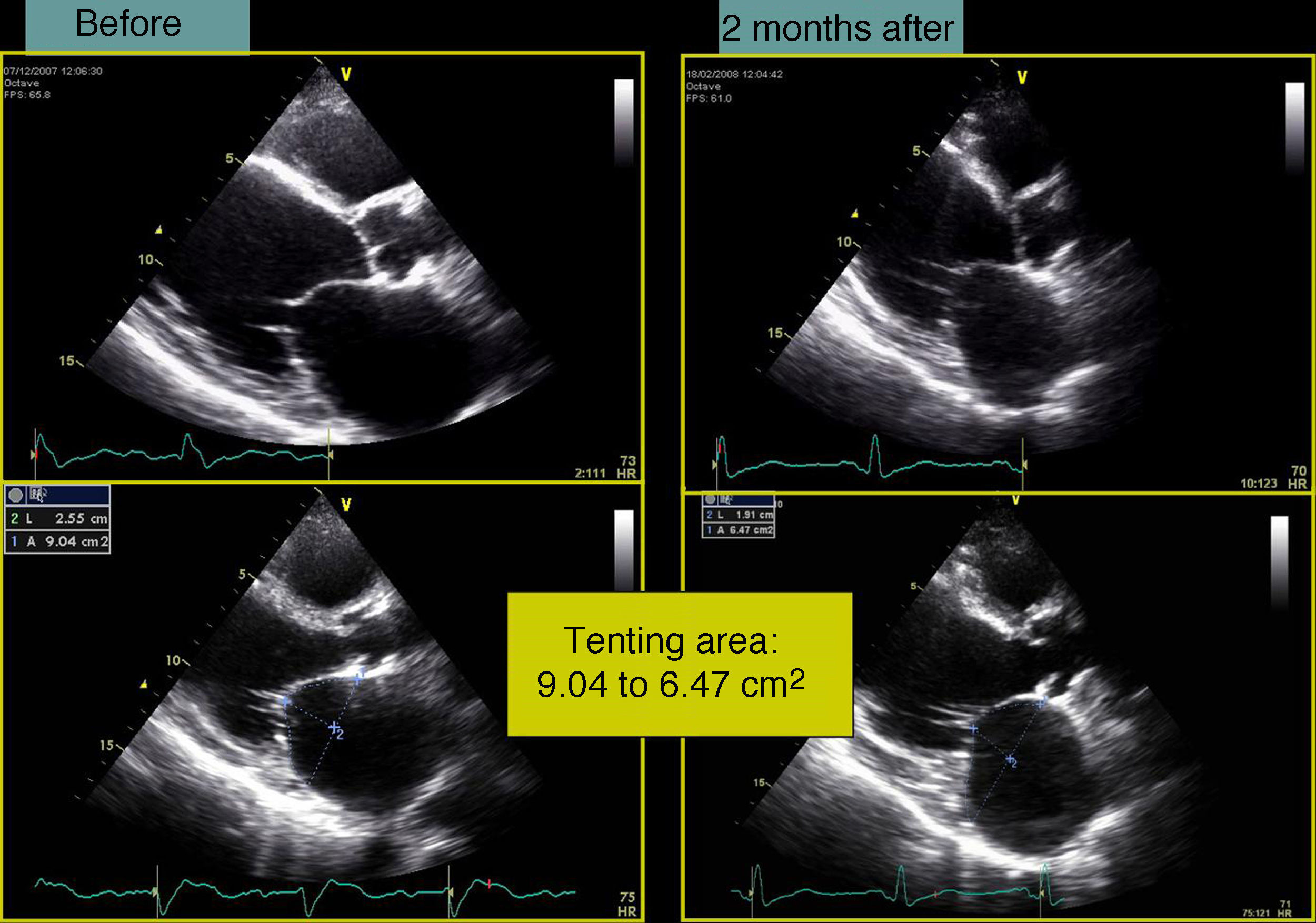
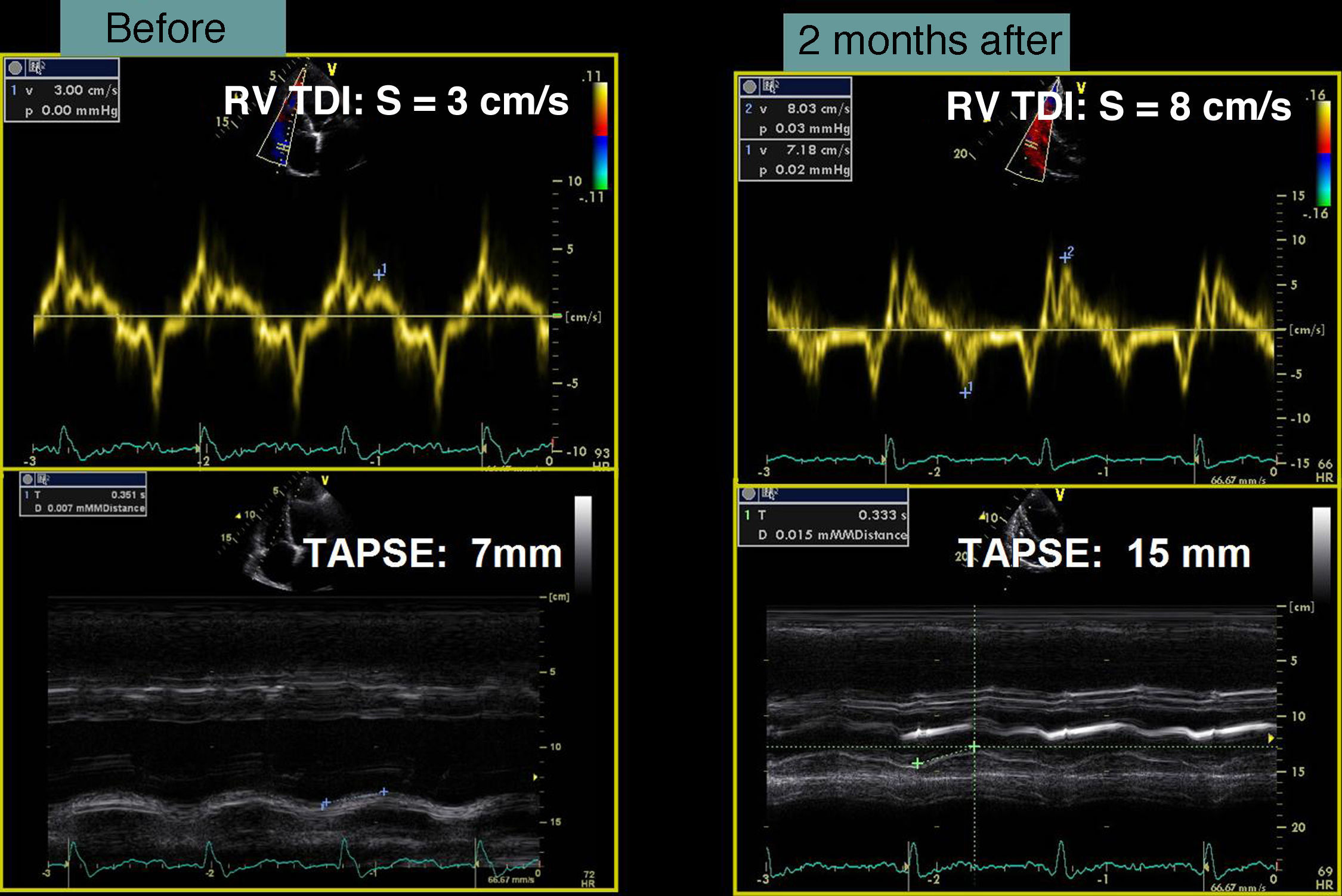
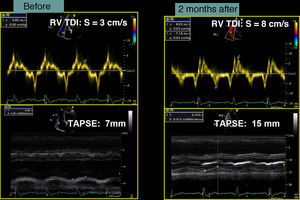
Case reportA 76-year-old man with a history of paroxysmal atrial fibrillation and anteroseptal myocardial infarction in 1996 underwent coronary artery bypass grafting (CABG) in 1997 (left internal mammary artery to left anterior descending artery, saphenous vein to first obtuse marginal branch, and saphenous vein to posterior descending artery). In the previous six months he had been hospitalized twice for worsening HF, once with acute pulmonary edema. He was in NYHA functional class III/IV under optimal medical therapy (maximum tolerated doses of angiotensin-converting enzyme inhibitors, beta-blockers, diuretics and statins. During this hospitalization he was without angina, but had symptoms of low cardiac output, which was treated with intravenous levosimendan, without success. No arrhythmias were observed and his heart rate remained stable at under 70bpm. Cardiac auscultation revealed a third sound with a I–II/VI systolic murmur at the apex, while bibasal rales compatible with pulmonary congestion were heard on pulmonary auscultation. There was also jugular distension (jugular venous pressure of 3cm at 30°) and malleolar edema. Laboratory tests showed hemoglobin 13.2g/dl, NT-proBNP 2275pg/ml, urea 59mg/dl and creatinine 1.4mg/ml; liver tests were normal, with SGOT and SGPT of 27 and 42U/l respectively. The electrocardiogram showed a pattern of anterolateral scarring and left bundle branch block, with QRS of 130ms. The echocardiogram revealed a moderately dilated left ventricle (end-diastolic and end-systolic dimensions of 75mm and 61mm, respectively, and end-diastolic and end-systolic volumes of 186ml and 133ml, respectively), moderate to severe global systolic dysfunction (EF by Simpson's biplane method: 34%) due to akinesia of the anterior septum, inferior wall and apical segments, and moderate posterior hypokinesia. The mitral valve showed no morphological alterations, but tenting (9cm2 in area and 26mm in height) and symmetrical stretching of the subvalvular apparatus were observed, leading to severe functional mitral regurgitation (jet area/left atrial area 45%, effective regurgitant orifice [ERO] area 0.4cm2, and regurgitant volume 43ml); there was moderate to severe PH (estimated pulmonary artery systolic pressure [PASP] 63mmHg) and right ventricular systolic dysfunction as assessed by tricuspid annular plane systolic excursion (TAPSE) of 7mm.
Given the presence of severe mitral regurgitation, although only functional, together with PH and right ventricular dysfunction, mitral valve annuloplasty was considered. Coronary angiography was accordingly performed which revealed patency of the three grafts and no progression of disease in the native circulation.
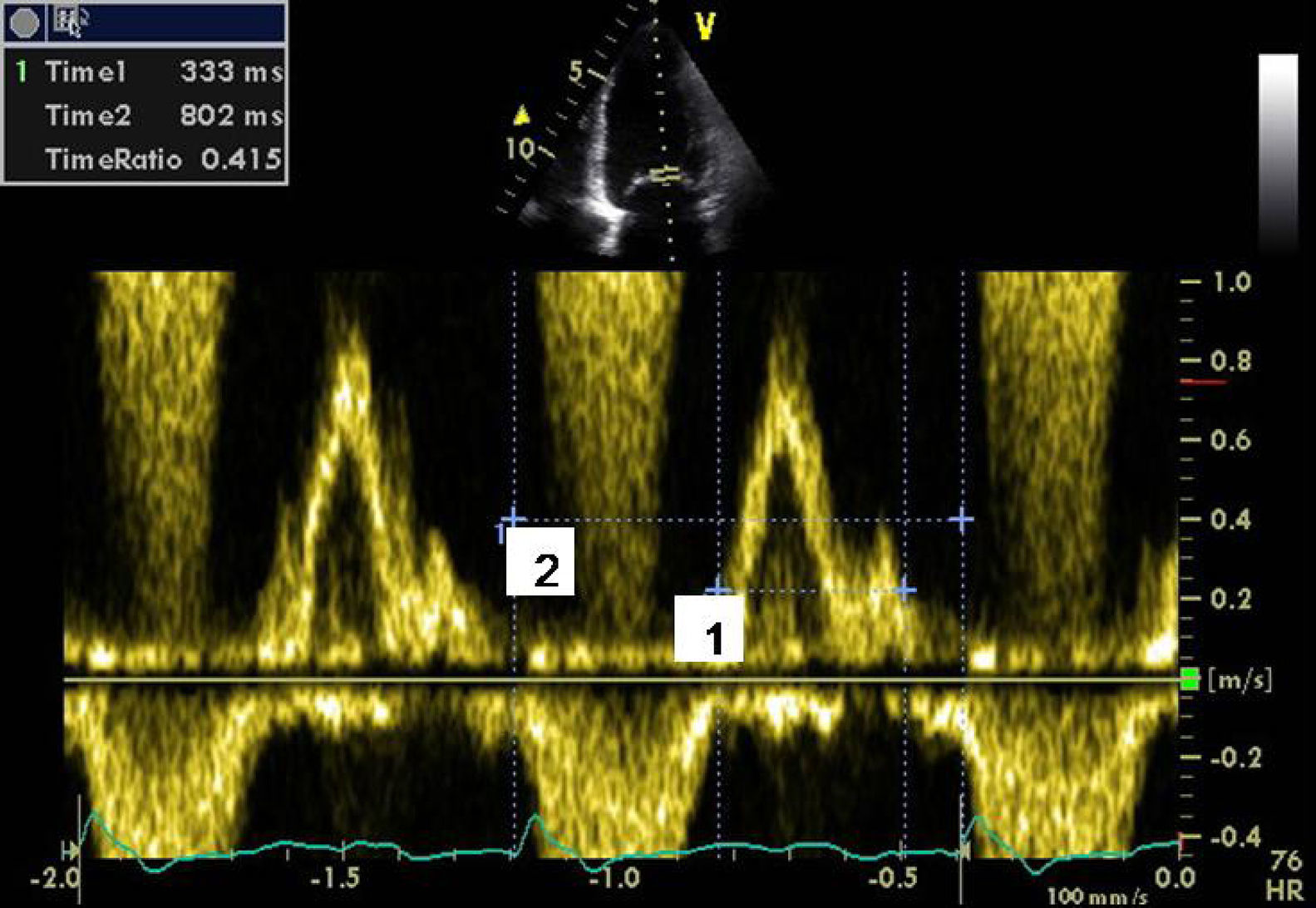
Bearing in mind the conflicting results of mitral reduction annuloplasty when not associated with revascularization,8–12 it was decided to evaluate the degree of mechanical dyssynchrony, even though right ventricular dysfunction, severe PH and grade >3 mitral regurgitation are considered predictors of non-response to CRT. Standard echocardiographic techniques as well as tissue Doppler and two-dimensional strain (2DS) with speckle tracking were used to evaluate atrioventricular (AV), interventricular and intraventricular synchrony. The following were observed:
- (1)
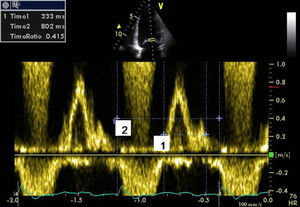
Absence of AV dyssynchrony (diastolic time of transmitral flow >40% of cardiac cycle) (Figure 1), and absence of presystolic mitral regurgitation;
- (2)
Presence of interventricular dyssynchrony evaluated by spectral Doppler (difference between pre-ejection times of pulmonary and aortic flow >45ms) (Figure 2);
- (3)
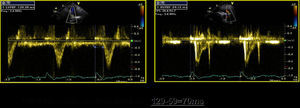
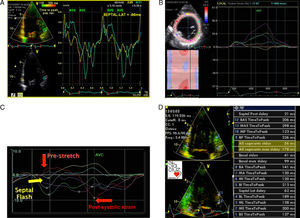
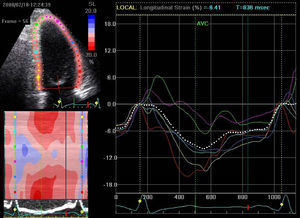
Presence of the multiphasic septal motion termed septal flash (Figure 3A–C), detected visually and quantified by speckle tracking as predominantly radial dyssynchrony, and also identified by analysis of longitudinal strain in apical views. This is caused by early septal contraction during isovolumic contraction, together with prestretching of the contralateral wall which presents late peak contraction (post-systolic strain). The time difference between peak systolic septal and lateral strain met the criteria for radial (>130ms) and longitudinal (>65ms) dyssynchrony by 2DS. Evaluation of longitudinal dyssynchrony by tissue synchronization imaging (TSI) was inconclusive, since comparison of peak systolic velocity of the contralateral basal segments showed no mechanical delay, even though the standard deviation of the twelve basal and mid segments in three apical views (Yu index) was >32ms (Figure 3D).
Figure 3.A TDI/TSI showing absence of intraventricular longitudinal dyssynchrony: difference of <65ms in peak systolic Q septal versus lateral inferior tissue velocity (56ms). (B and C) Early septal contraction (septal flash: early systolic curves) and peak lateral strain after aortic valve closure: difference of >130ms between post-systolic strain (late curves) and peak septal strain assessed by radial strain in parasternal view and longitudinal strain in apical 4-chamber view. The characteristic curves of radial and longitudinal strain seen in septal flash can be identified: early septal contraction (septal flash), early systolic pre-stretching (absence of contraction=stretching) of the lateral wall with peak strain after aortic valve closure (post-systolic strain). (D) Yu index: standard deviation of the 12 basal and mid segments (apical 4-chamber, apical 2-chamber and apical long-axis views) of >32ms.
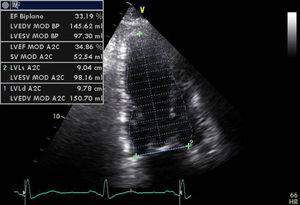
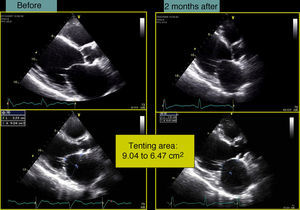
In our experience, and in agreement with previous studies,13–15 septal flash is a predictor of response to CRT with high specificity and sensitivity. In the case presented, after weighing the risks and benefits of surgery versus resynchronization therapy, we opted for CRT-ICD implantation. The patient was discharged four days later and returned to normal activity; at clinical reassessment after one month he reported that he had returned to normal life, walking 5km a day, and his furosemide dosage was reduced from 120 to 20mg/day. Two months later, echocardiographic reassessment showed reverse remodeling, with >15% reduction in ventricular volumes (end-diastolic volume 186–150ml, end-systolic volume 133–98ml) (Figure 4, Videos 1A and 1B) and a marked reduction in mitral tenting area (9.04–6.47cm2) (Figure 5), with mitral regurgitation now mild (grade 1). PASP had decreased to 39mmHg, left atrial volume had decreased significantly from 34 to 28cm2, and right ventricular function had improved, with TAPSE increasing from 7 to 15mm (Figure 6). No residual mechanical dyssynchrony was observed; no septal flash was detected either visually or by M-mode echocardiography or quantified by radial and longitudinal strain (Figure 7). These echocardiographic and clinical results remained the same over 2 years of follow-up.
Echocardiography is playing an increasingly important role in the study of patients with clinical and echocardiographic criteria for cardiac resynchronization therapy. Clinical trials on CRT have consistently identified a population of patients who are non-responders (20–30%), which indicates a need to improve selection criteria, to define more precisely the best left ventricular lead implantation site, and to optimize the device's AV and VV intervals on the basis of evaluation of mechanical dyssynchrony by imaging techniques. No single parameter of mechanical dyssynchrony has yet been identified that can predict those who will or will not respond.8 However, echocardiography is able to detect, locate and quantify the extent of dyssynchrony, using various parameters of AV, interventricular and intraventricular dyssynchrony. To quantify intraventricular dyssynchrony, longitudinal and radial myocardial strain as well as velocities must be assessed. Several studies5–7,16 have shown that the greater the extent and degree of mechanical dyssynchrony, the greater the likelihood of response to CRT. A more recent multicenter study involving three European centers showed that the presence of septal flash, particularly when associated with AV and/or interventricular dyssynchrony, identifies responders to CRT with sensitivity and specificity approaching 100%. Even more importantly, the absence of any mechanical dyssynchrony (AV, interventricular or intraventricular) identifies non-responders with a high degree of sensitivity and specificity.13
There are no universally accepted criteria to define CRT response. Some studies use strictly clinical criteria (reduction of at least one NYHA class together with improvement of ≥25% in the 6-min walk test), some require a structural response (left ventricular reverse remodeling and reduction of at least two grades in mitral regurgitation), while others combine clinical and structural criteria. The definition of left ventricular remodeling has been established as a reduction in ventricular volumes, particularly end-systolic volume of >15%, together with improvement in functional mitral regurgitation resulting from normalization of ventricular geometry and hence reduction in tenting area.17–19
In the CARE-HF study, reduction in mitral regurgitation, as shown by reduced ERO area resulting from improved ventricular function, was the main prognostic marker in these patients.19
Surgical treatment of functional mitral regurgitation in the context of ischemic dilated cardiomyopathy improves symptoms when associated with CABG, but only initially; it does not improve functional status or long-term survival.8–11
The central issue in the case presented was functional mitral regurgitation in the context of ischemic cardiomyopathy, with no active ischemia. Mitral regurgitation was assumed to be the cause of the patient's episodes of decompensation and hospitalization for acute pulmonary edema. Mortality in such patients increases in proportion to the severity of mitral regurgitation, irrespective of the severity of ventricular dysfunction.12
The patient met the criteria in the guidelines for surgical treatment of mitral regurgitation (class IIB recommendation, level of evidence C), with a EuroSCORE surgical risk of 32.6% based on the fact that he had been revascularized and currently had no evidence of ischemia.
At the same time, he also met the criteria for CRT (class I recommendation, level of evidence A).4 There was, however, right ventricular dysfunction and significant pulmonary hypertension, which have been identified in some clinical trials, particularly that by Díaz-Infante et al.,20 as predictors of non-response to CRT. The latter study also identified coronary disease, severe mitral regurgitation and left ventricular end-diastolic dimension >75mm as important predictors of non-response to CRT, factors that our patient also presented. Although there have been no multicenter studies that include assessment of mechanical dyssynchrony in the selection of patients for CRT, several studies in tertiary centers have demonstrated the importance of echocardiographic evaluation of the presence and degree of mechanical dyssynchrony.21 A recent study highlighted the importance of septal flash in quantifying dyssynchrony and in predicting short- and long-term response. As septal flash is a manifestation of intraventricular dyssynchrony, an angle-independent method is necessary that does not require the image to be aligned. With the new echocardiographic technique of speckle tracking, radial myocardial strain can be quantified, which is not possible with tissue Doppler imaging (TDI) or TSI. Speckle tracking is also excellent for characterizing the pattern of ventricular synchrony, providing typical strain curves.13
The case presented highlights the importance of intraventricular radial dyssynchrony, identified by two-dimensional and 2DS echocardiography, as a predictor of an excellent clinical and echocardiographic response to CRT. Observational studies have shown that intraventricular dyssynchrony is a significant factor determining response to CRT, while interventricular dyssynchrony appears to be less important. A study by Gorcsan et al.14 found that patients with longitudinal dyssynchrony on TDI and radial dyssynchrony on speckle tracking are more likely to be responders. Isolated intraventricular radial dyssynchrony, or septal flash, has been identified as a predictor of left ventricular reverse remodeling.15
The strongest predictor of response to CRT is in fact dyssynchrony at all three levels: AV, interventricular and intraventricular. In the case presented AV dyssynchrony was absent and analysis of intraventricular longitudinal dyssynchrony was inconclusive due to the absence of one of the standard criteria, i.e. septal-lateral longitudinal dyssynchrony, but a positive Yu index. The latter frequently gives false positives in ischemic cardiomyopathy with wall or segmental scarring. In the case presented, the patient had interventricular and intraventricular radial dyssynchrony visualized on two-dimensional echocardiography and characterized and quantified by two-dimensional strain imaging.
In conclusion, echocardiographic evaluation is crucial in patients referred for CRT, especially when there are doubts, and should be comprehensive, including both morphology and function. It is particularly important to choose the best techniques to identify and characterize mechanical dyssynchrony: spectral Doppler for AV and interventricular synchrony and 2DS for predominantly radial synchrony due to its angle-independent nature.
Conflict of interestThe authors have no conflicts of interest to declare.
Supplementary material associated with this article can be found in the online version available at doi:10.1016/j.repce.2011.10.004.
Please cite this article as: Gomes, R. Sucesso da terapêutica de ressincronização cardíaca num doente com insuficiência cardíaca e regurgitação mitral secundária a doença isquémica: Importância do Septal flash. Rev Port Cardiol. 2011; 30(11):855–861.