Spontaneous coronary artery dissection (SCAD) is an unusual cause of acute coronary syndrome (ACS). Better recognition and diagnosis has raised awareness of this condition. However, the pathophysiology of SCAD and its prognosis are still little understood. We aimed to investigate the characteristics and prognosis of patients with SCAD, and subsequently performed a review of literature.
MethodsSingle-center, retrospective study performed in patients hospitalized from January 2010 to December 2016 with suspected ACS (n=5002) whose final diagnosis was SCAD (n=27; 0.5%).
ResultsPatients with SCAD were mainly female (81.5%; n=22), with median age of 56. Predisposing factors were identified in 12 (44%) patients and precipitating factors in three (11.1%). Non-ST elevation myocardial infarction (NSTEMI) was the main form of presentation (51.9%). The left anterior descending artery (LAD) territory was the most commonly involved (n=12, 44.4%). Type 2 dissection was the most prevalent angiographic pattern (n=17, 63%). The majority of patients (n=15; 55.6%) were managed medically and the remaining patients underwent percutaneous coronary intervention (PCI) with drug-eluting stents. Seven patients re-infarcted while in the hospital. Over the median follow-up period of 20 months, 7.4% of patients (n=2) had symptoms of heart failure (HF) and 14.8% developed ACS (in three patients the event occurred in a coronary territory other than that of the index case, and in one patient it occurred in the previously affected territory). There were no deaths.
ConclusionIn the studied population, SCAD was more prevalent in middle-aged women. Despite the high prevalence of in-hospital re-infarction or during follow-up, the prognosis was good overall.
A disseção coronária espontânea (DCE) é uma causa infrequente de síndrome coronária aguda. O crescente reconhecimento e diagnóstico dessa entidade tem alertado os clínicos para a sua ocorrência. Porém, a fisiopatologia da DCE e o respetivo prognóstico ainda se encontram mal esclarecidos. O objetivo deste estudo foi investigar as características e o prognóstico dos doentes com DCE, efetuando-se, posteriormente, uma revisão da literatura.
MétodosEstudo retrospetivo, unicêntrico, realizado em doentes admitidos de janeiro de 2010 a dezembro de 2016, sob a suspeita de SCA (n = 5002), cujo diagnóstico final foi DCE (n = 27; 0,5%).
ResultadosOs doentes com DCE eram na sua maioria mulheres (81,5%; n = 22), com mediana de 56 anos. Em 12 doentes (44%) foram identificados fatores predisponentes e em três (11,1%) fatores precipitantes. O enfarte sem supradesnivelamento do segmento ST foi a forma mais frequente de apresentação (51,9%), o território da descendente anterior foi o mais frequentemente envolvido (n = 12, 44,4%). O padrão angiográfico de dissecção tipo 2 (n = 17, 63%) foi o mais prevalente. A maioria dos doentes (n = 15; 55,6%) foi tratada medicamente, os restantes fizeram angioplastia com stents revestidos com fármaco. Durante a hospitalização, sete doentes tiveram re-enfarte. Durante o período mediano de 20 meses de seguimento, 7,4% (n = 2) dos doentes apresentaram sintomas de insuficiência cardíaca e 14,8% apresentaram SCA (em três doentes o evento ocorreu num território coronário diferente do caso índex e em um doente no território coronário previamente afetado). Não ocorreram eventos mortais.
ConclusãoNa população estudada, a disseção coronária espontânea foi mais prevalente nas mulheres de meia-idade. Apesar da prevalência de re-enfarte durante e após a hospitalização não ser insignificante, o prognóstico, globalmente, foi bom.
Acute coronary syndrome
Connective tissue disease
Diameter
Dimensions
Heart failure
Hormone therapy
Left anterior descending artery
Left circumflex artery
Left main coronary artery
Myocardial infarction
Multiparity
Non-ST elevation myocardial infarction
Percutaneous coronary intervention
Physical stress
Right coronary artery
Sudden cardiac arrest
Spontaneous coronary artery dissection
Systemic inflammatory disease
ST-elevation myocardial infarction
Spontaneous coronary artery dissection (SCAD) is defined as a non-traumatic, non-iatrogenic separation of the coronary artery walls, creating a false lumen.1 Traditionally thought of as rare, for many years only anecdotal cases were reported. However, in recent years, probably since the increased use of coronary angiography and intracoronary imaging techniques in acute coronary syndromes (ACS), it has been recognized as an important cause of myocardial infarction, especially in women.2–4 The true prevalence of SCAD in the general population is unfortunately unknown because of under-diagnosis. However, based on modern series, its prevalence in ACS is 1.7-4%.5,6 This study aimed to investigate the characteristics, clinical presentation, therapeutic approach and outcome in patients with ACS presenting with SCAD over a period of seven years.
MethodsThis was a descriptive and retrospective analysis of patients admitted to a Cardiology Department over seven consecutive years, from January 2010 to December 2016, with a diagnosis of ACS due to SCAD. SCAD was diagnosed in the presence of angiographic characteristics on coronary angiography and was categorized according to the Saw classification7 as follows: Type 1, contrast dye staining of the artery wall with multiple radiolucent lumens (the pathognomonic angiographic appearance of SCAD); Type 2, diffuse stenosis of varying severity and length (typically longer than 20 mm), with frequent subtle abrupt changes in the artery caliber from normal diameter to diffuse smooth narrowing. This diffuse narrowing may be bordered by normal artery segments, proximally and distally (type 2A variant) or it may extend to the apical tip of the artery (type 2B variant). Lastly, Type 3, focal or tubular (typically less than 20 mm) stenosis that mimics atherosclerosis and requires intracoronary imaging to confirm diagnosis.
Clinical, angiographic, therapeutic, and follow-up data up to the end of December 2016 were analyzed. Data were obtained from medical records of hospital stay and subsequent consultations.
ResultsBaseline patient characteristicsOf a total of 5002 patients admitted to our institution with suspected ACS from January 2010 to December 2016, 27 (0.5%) received a final diagnosis of SCAD (Table 1). The median age of the cohort was 56±11 years (maximum 82 and minimum 38), and the patients were predominantly female (81.5%; n=22), with 17 being post-menopausal. Hypertension (55.6%; n=15) and dyslipidemia (44.4%; n=12) were the most prevalent cardiovascular risk factors, followed by active smoking (18.5%; n=5). Only two patients had previous history of coronary artery disease.
Patient characteristics, clinical presentation, treatment and follow-up.
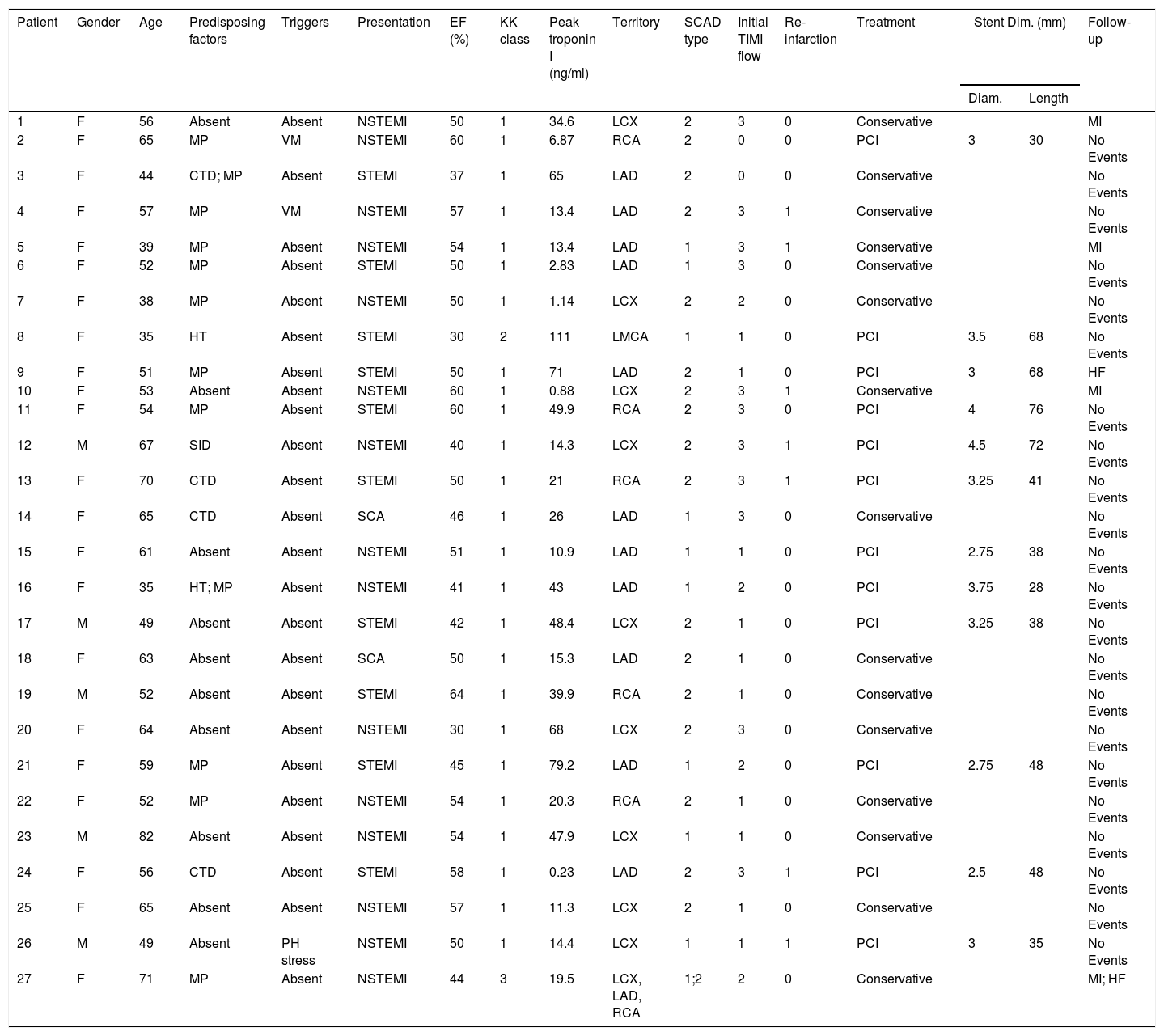
| Patient | Gender | Age | Predisposing factors | Triggers | Presentation | EF (%) | KK class | Peak troponin I (ng/ml) | Territory | SCAD type | Initial TIMI flow | Re-infarction | Treatment | Stent Dim. (mm) | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diam. | Length | |||||||||||||||
| 1 | F | 56 | Absent | Absent | NSTEMI | 50 | 1 | 34.6 | LCX | 2 | 3 | 0 | Conservative | MI | ||
| 2 | F | 65 | MP | VM | NSTEMI | 60 | 1 | 6.87 | RCA | 2 | 0 | 0 | PCI | 3 | 30 | No Events |
| 3 | F | 44 | CTD; MP | Absent | STEMI | 37 | 1 | 65 | LAD | 2 | 0 | 0 | Conservative | No Events | ||
| 4 | F | 57 | MP | VM | NSTEMI | 57 | 1 | 13.4 | LAD | 2 | 3 | 1 | Conservative | No Events | ||
| 5 | F | 39 | MP | Absent | NSTEMI | 54 | 1 | 13.4 | LAD | 1 | 3 | 1 | Conservative | MI | ||
| 6 | F | 52 | MP | Absent | STEMI | 50 | 1 | 2.83 | LAD | 1 | 3 | 0 | Conservative | No Events | ||
| 7 | F | 38 | MP | Absent | NSTEMI | 50 | 1 | 1.14 | LCX | 2 | 2 | 0 | Conservative | No Events | ||
| 8 | F | 35 | HT | Absent | STEMI | 30 | 2 | 111 | LMCA | 1 | 1 | 0 | PCI | 3.5 | 68 | No Events |
| 9 | F | 51 | MP | Absent | STEMI | 50 | 1 | 71 | LAD | 2 | 1 | 0 | PCI | 3 | 68 | HF |
| 10 | F | 53 | Absent | Absent | NSTEMI | 60 | 1 | 0.88 | LCX | 2 | 3 | 1 | Conservative | MI | ||
| 11 | F | 54 | MP | Absent | STEMI | 60 | 1 | 49.9 | RCA | 2 | 3 | 0 | PCI | 4 | 76 | No Events |
| 12 | M | 67 | SID | Absent | NSTEMI | 40 | 1 | 14.3 | LCX | 2 | 3 | 1 | PCI | 4.5 | 72 | No Events |
| 13 | F | 70 | CTD | Absent | STEMI | 50 | 1 | 21 | RCA | 2 | 3 | 1 | PCI | 3.25 | 41 | No Events |
| 14 | F | 65 | CTD | Absent | SCA | 46 | 1 | 26 | LAD | 1 | 3 | 0 | Conservative | No Events | ||
| 15 | F | 61 | Absent | Absent | NSTEMI | 51 | 1 | 10.9 | LAD | 1 | 1 | 0 | PCI | 2.75 | 38 | No Events |
| 16 | F | 35 | HT; MP | Absent | NSTEMI | 41 | 1 | 43 | LAD | 1 | 2 | 0 | PCI | 3.75 | 28 | No Events |
| 17 | M | 49 | Absent | Absent | STEMI | 42 | 1 | 48.4 | LCX | 2 | 1 | 0 | PCI | 3.25 | 38 | No Events |
| 18 | F | 63 | Absent | Absent | SCA | 50 | 1 | 15.3 | LAD | 2 | 1 | 0 | Conservative | No Events | ||
| 19 | M | 52 | Absent | Absent | STEMI | 64 | 1 | 39.9 | RCA | 2 | 1 | 0 | Conservative | No Events | ||
| 20 | F | 64 | Absent | Absent | NSTEMI | 30 | 1 | 68 | LCX | 2 | 3 | 0 | Conservative | No Events | ||
| 21 | F | 59 | MP | Absent | STEMI | 45 | 1 | 79.2 | LAD | 1 | 2 | 0 | PCI | 2.75 | 48 | No Events |
| 22 | F | 52 | MP | Absent | NSTEMI | 54 | 1 | 20.3 | RCA | 2 | 1 | 0 | Conservative | No Events | ||
| 23 | M | 82 | Absent | Absent | NSTEMI | 54 | 1 | 47.9 | LCX | 1 | 1 | 0 | Conservative | No Events | ||
| 24 | F | 56 | CTD | Absent | STEMI | 58 | 1 | 0.23 | LAD | 2 | 3 | 1 | PCI | 2.5 | 48 | No Events |
| 25 | F | 65 | Absent | Absent | NSTEMI | 57 | 1 | 11.3 | LCX | 2 | 1 | 0 | Conservative | No Events | ||
| 26 | M | 49 | Absent | PH stress | NSTEMI | 50 | 1 | 14.4 | LCX | 1 | 1 | 1 | PCI | 3 | 35 | No Events |
| 27 | F | 71 | MP | Absent | NSTEMI | 44 | 3 | 19.5 | LCX, LAD, RCA | 1;2 | 2 | 0 | Conservative | MI; HF | ||
CTD: connective tissue disease; Diam.: diameter; Dim.: dimensions; EF: ejection fraction; HF: heart failure; HT: hormone therapy; KK: Killip-Kimball classification; LAD: left anterior descending artery; LCX: left circumflex artery; LMCA: left main coronary artery; MI: myocardial infarction; MP: multiparity; NSTEMI: non-ST elevation myocardial infarction; PCI: percutaneous coronary intervention; PH stress: physical stress; RCA: right coronary artery; SCA: sudden cardiac arrest; SCAD: spontaneous coronary artery dissection; SID: systemic inflammatory disease; STEMI: ST-elevation myocardial infarction; TIMI: thrombolysis in myocardial infarction; VM: valsalva maneuver type activity.
In 17 patients (63%), predisposing factors were identified; such as connective tissue disease in 14.8% (n=4); systemic inflammatory disease (SID) in 3.7% (n=1); hormone therapy (HT) in 11.5% (n=3) of the total cohort; and multiparity in 56.5% of the females.
As precipitating factors, intense exercise was documented in one patient and Valsalva-type activities in two.
The predominant symptom was chest discomfort, reported in 96.3% of patients.
On admission, 51.9% of patients had NSTEMI, 37.0% ST-elevation myocardial infarction, 7.4% cardiac arrest and 3.7% unstable angina. During hospital stay, the average troponin I peak reached was 31.47±28.4 ng/ml. Two thirds of patients (n=18; 66.7%) had normal left ventricular systolic function and 92.6% (n=25) recovered with no signs of HF.
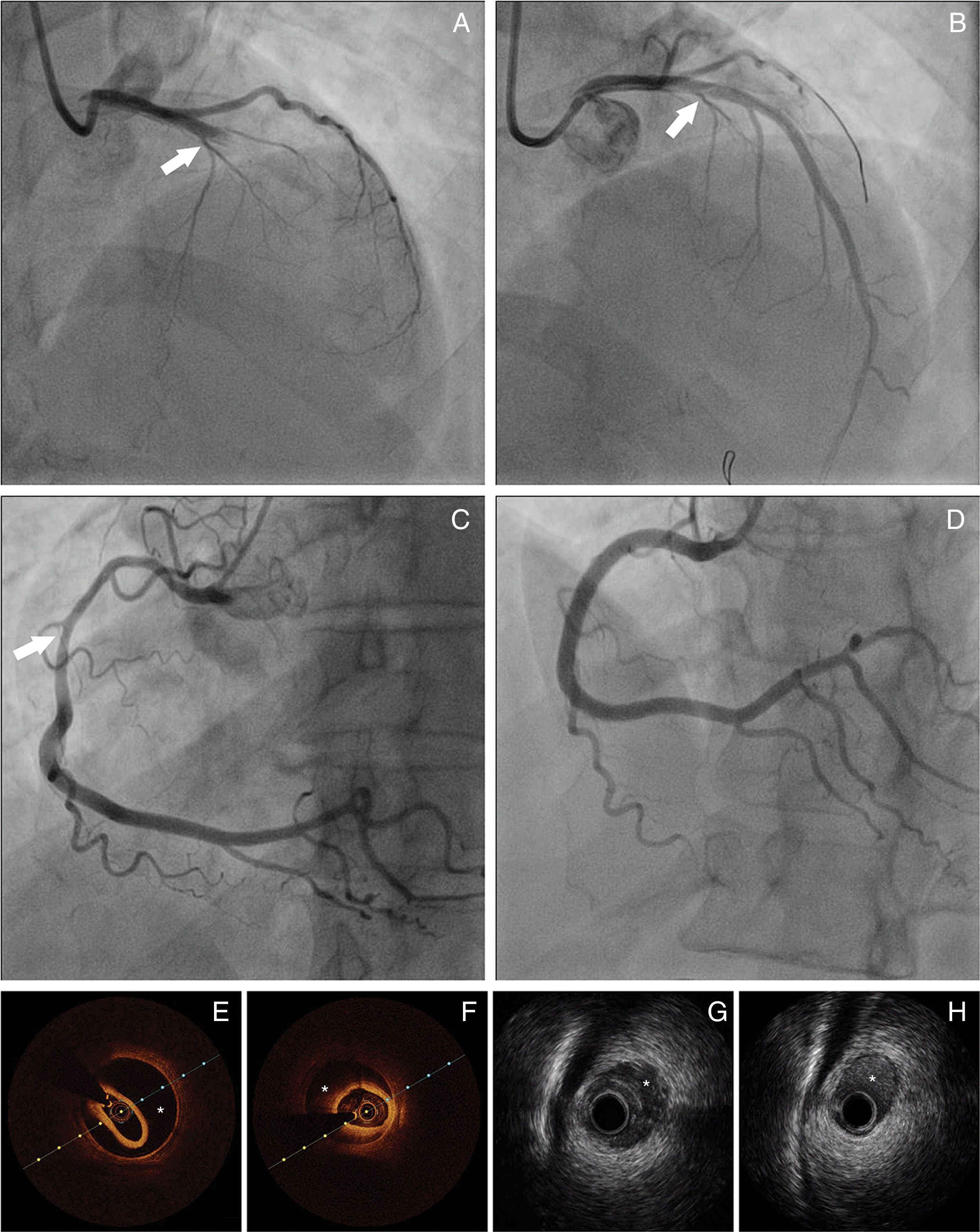
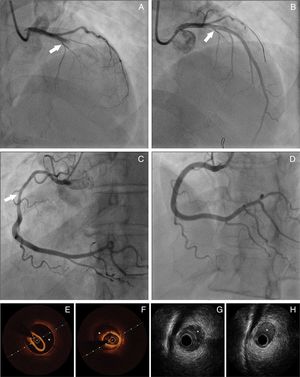
Treatment and in-hospital proceduresOn coronary angiography, severe coronary tortuosity (defined as two consecutive 180-degree turns by visual estimation in a major epicardial artery) was identified in one third of patients (n=8; 29.6%). Diagnosis was enabled by coronary angiography alone for the majority of cases (92.5%; n=25). Intracoronary imaging (optical coherence tomography [OCT]/intravascular ultrasound [IVUS]) was only performed in two patients (Figure 1). In general, only one artery was identified with SCAD, apart from one case, where dissection was found in multiple territories (diagonal, obtuse marginal and right coronary arteries [RCA]). The territory of the LAD was the most commonly involved (n=13), followed by the circumflex artery (n=10) and RCA (n=5). Only one patient had dissection of the left main coronary artery (LMCA). The most common angiographic pattern was type 2 dissection (n=17; 63%), followed by type 1 (n=10; 37%), and 48.1% (n=13) of patients had initial thrombolysis in myocardial infarction (TIMI) flow 0-1.
Panel A: Coronary angiography from patient 8 – spontaneous coronary artery dissection (SCAD) type 1, extending from left main coronary artery to left anterior descending artery (LAD). Panel B: Coronary angiography from patient 8 showing the result obtained after guidewire advancement to the true LAD lumen. Panel C: Coronary angiography from patient 11 showing diffuse lumen narrowing involving the proximal and mid segments of right coronary artery – SCAD type 2. Panel D: Coronary angiography from patient 11 showing the angiographic result after stent implantation. Panels E-F: Optical coherence tomography from patient 22 of distal segment of right coronary artery, depicting intramural hematoma (asterisk). Panels G-H: Cross-sectional intravenous ultrasound views from patient 9 depicting intramural hematoma (asterisk) causing luminal stenosis in the mid-LAD.
Patients with TIMI flow 3 or whose vessels were thin, were treated with dual antiplatelet therapy, beta blockers, angiotensin converting enzyme (ACE) inhibitors and statins. If SCAD was identified on angiography, heparin was immediately stopped. About 44.4% of patients (n=12) underwent angioplasty with drug-eluting stents; two had two stents implanted. The artery dimension covered by the stent was 49 mm on average [25th percentile: 36; 75th percentile: 68] lengthwise and 3.27 mm [25th percentile: 2.8; 75th percentile: 3.7] in diameter. None of the patients required coronary artery bypass grafting (CABG).
In-hospital adverse eventsDuring hospital stay, seven patients (25.9%) suffered re-infarction, an average of six days after the index event. Recurrence of SCAD was found in four patients. The initial treatment strategy was only modified in two patients who suffered re-infarction; they underwent coronary angioplasty. No deaths were recorded.
Follow-up and adverse eventsOver a median 20-month follow-up period, four patients (14.8%) had a myocardial infarction, an average of 222 days post-index event. Of these, three had a new SCAD event in a different territory. One had recurrence in a native vessel that was previously treated medically. There were no cases of stent thrombosis during follow-up.
Symptoms of HF with a maximum New York Heart Association class II were observed in two patients (7.4%). No deaths were recorded.
DiscussionSpontaneous coronary artery dissection is uncommon and often misdiagnosed. Diagnosis can be challenging, with a high degree of clinical suspicion necessary.7
The underlying etiology of SCAD appears to be multifactorial and it is believed to be triggered by a precipitating stressor in an individual with underlying predisposing arterial disease.2
A variety of potential predisposing factors have been described, including fibromuscular dysplasia, pregnancy, connective tissue disorders, SID, HT and coronary artery spasm. A predisposing factor was identified in two fifths of our population. In relation to arterial integrity, the Eleid et al. study showed that tortuosity is present in a higher proportion of patients with SCAD and repeat dissections were common within tortuous segments.8 In fact, we found this anatomical variation in a fifth of our cases, but whether arterial tortuosity predisposes to arterial fragility or is only a marker of SCAD remains undetermined. In our population, the only precipitating factors identified were intense physical exercise and Valsalva-type activities. However, other factors, like intense emotional stress, labor and delivery, use of recreational drugs and intense HT have been described.
Hence, in view of the potential association between SCAD and systemic disorders, clinicians should thoroughly investigate to find underlying causes.
The clinical presentation of SCAD varies from asymptomatic to sudden death, but a large proportion of patients present with ACS.9–11 In our population, ACS was the main form of presentation. It can affect one or more coronary arteries, but, as in our study, is most common in the anterior descending artery.12
Coronary angiography is widely available and is the gold standard for imaging in patients with SCAD. Nonetheless, this examination is notoriously suboptimal to visualize intimal tears, as it only provides a two-dimensional image.
There are three main patterns of SCAD2: type 1 angiographic SCAD appearance which, although not the most common pattern, can be demonstrated well on angiography with multiple radiolucent and contrast dye stains in the artery wall; type 2 angiographic SCAD appearance, which is the most common, with long diffuse stenosis of varying severity, commonly in mid to distal segments (this pattern was observed in about two-thirds of our series); and type 3 angiographic pattern, which is more challenging to diagnosis, because it mimics atherosclerosis (not seen in our population).
Complementary techniques such as IVUS and OCT contribute to better identification and classification of SCAD. IVUS has a lower spatial resolution (150-200 μm), but has deeper penetration, allowing full vessel wall visualization. It can determinate the true and false lumen and detect intramural hematoma (IMH) in its full extension.13 Although the poorer penetration of OCT hampers visualization of the full extent of IMHs, it is more sensitive, has much higher resolution (10-20 μm) and can visualize intimal tears, and true and false lumens. It is also superior in visualizing strut apposition, allowing stent optimization if angioplasty is performed.4,14-16 We only used these tools to confirm diagnosis or to guide SCAD treatment in two procedures, as we only recently acquired the equipment.
There are no prospective randomized studies that specifically address the management of SCAD, so its ideal management remains unclear. In terms of medical therapy, there is a general consensus that beta blockers play an important role. They reduce artery wall stress and should therefore be used acutely and long-term following SCAD. The role of antiplatelet therapy for patients with SCAD not treated with stenting is less clear. Based on the benefits of aspirin in acute coronary syndrome and its relatively safe profile, it would appear to be a reasonable option for acute and long-term treatment. As a proportion of SCAD involves intimal tears that can be pro-thrombotic, in theory, double antiplatelet therapy could be beneficial, reducing the false lumen thrombus burden. Although evidence is lacking, clopidogrel is often administered for one to 12 months following SCAD and then discontinued. The role of novel P2Y12 has not been defined. Glycoprotein IIb/IIIa inhibitors are not recommended due to the potential risk of extending the dissection. Anticoagulation for SCAD is subject to debate and it should be discontinued as soon as the diagnosis of SCAD is made due to the potential risk of extending the dissection. Thrombolysis is harmful for SCAD and should be avoided. ACE inhibitors and angiotensin receptor blockers have not been studied in SCAD and tend to be administered in patients with left ventricular dysfunction. Statins should be prescribed if the patient has concomitant dyslipidemia.
The decision to bypass a dissected artery depends on the patient's hemodynamic status and coronary anatomy; site of dissection, the number of vessels involved, distal flow and the possibility of intervention.17,18 In stable patients with normal coronary flow, the conservative approach is preferable.17 This option relies on expert opinion based on observation that dissected arteries heal spontaneously in most cases.
Nonetheless, bypass should be considered in patients with high-risk features, such as ongoing/recurrent ischemia or chest pain, cardiogenic shock, ventricular arrhythmias (ventricular tachycardia or fibrillation) and LMCA dissection.2 Angioplasty is indicated in cases with suitable coronary anatomy (one-vessel disease), due to the high risk of propagation of the dissection related to the procedure17,19; there is still no ideal stent for the treatment of such lesions. We usually follow this rationale to decide which patients should undergo angioplasty. Due to the extent of the dissections, long stents or two stents were frequently used to cover the lesion. A long stent should be selected to provide adequate coverage of both lesion edges (at least 5-10 mm longer proximally and distally), in order to prevent extension of the IMH when compressed by the stent. PCI of dissected coronary arteries can be notoriously challenging and often ends with suboptimal results. First of all, it can be difficult to advance the guidewire through the distal true lumen; second, the IMH can propagate anterogradely or retrogradely with angioplasty; third, the dissections often involve distal coronary arteries, which are too small to implant stents; fourth, even if the dissected arteries are large, the dissections are often extensive, requiring long stents and thus increasing the risks of restenosis; last of all, IMHs resorb and heal over time, and this can result in strut malapposition, increasing the risk of very late stent thrombosis.
CABG may be appropriate in multi-vessel disease or when the LMCA is affected.17 The risk of the procedure is related to not identifying the true lumen. In the only case we had of LMCA dissection, due to the patient's hemodynamic instability and the impossibility to transfer the patient to an operating room within a few minutes, we opted to perform a PCI, the result of which was optimal.
After the acute phase, estimated survival is good, at about 70% to 90%.20 However, there is a risk of recurrence in 50% of patients.21,22
In view of the high rates of major adverse cardiac events in the long term, close follow-up is recommended for patients who suffer a SCAD. In patients treated by PCI, OCT should also be considered to assess for malapposed or uncovered struts before discontinuing dual antiplatelet therapy.
ConclusionIn our population, the prevalence of SCAD was 0.5% among patients who underwent catheterization for suspected ACS. It was more prevalent in middle-aged women. Although SCAD should be considered in the presence of ACS, sudden cardiac arrest could be one form of presentation. It can be associated with SID and patients should be suitably screened. Despite the high prevalence of re-infarction in-hospital or during follow-up, prognosis was good.
Conflicts of interestThe authors have no conflicts of interest to declare.


 SCAD) type 1, extending from left main coronary artery to left anterior descending artery (
SCAD) type 1, extending from left main coronary artery to left anterior descending artery (

