Systolic anterior motion (SAM) is a postoperative complication of mitral valve repair, with an incidence of 5–10%. Early recognition of the signs and symptoms of SAM is essential for the management of these patients. This article focuses on the pathophysiology and dynamics of SAM and the treatment strategies described in the literature. The authors present a case study and echocardiographic images illustrating the clinical relevance of the mechanism involved, in order to clarify whether surgical reintervention is necessary.
O movimento anterior sistólico (SAM) é uma complicação pós cirúrgica da valvuloplastia mitral, sendo a sua incidência de 5-10%. O reconhecimento precoce dos sinais e sintomas de SAM é imperativo no delinear de estratégia terapêutica nesses pacientes. Este artigo foca os principais mecanismos fisiopatológicos do SAM dinâmico e modalidades de tratamento descritas na literatura. Os autores descrevem um caso clínico e as imagens ecocardiográficas captadas ilustrando a relevância clínica do mecanismo envolvido, na tentativa de esclarecer uma questão suscitada: reintervenção cirúrgica necessária?
Systolic anterior motion (SAM) is due to partial obstruction of the left ventricular outflow tract (LVOT) by the mitral valve (MV) anterior leaflet.1 It has been reported in patients with hypertrophic cardiomyopathy, following myocardial infarction, and as a postoperative complication of MV repair.2,3
Case reportWe describe the case of a 71-year-old female patient, Caucasian, admitted to our department for decompensated heart failure (NYHA class III/IV). Her personal history included hypertension, dyslipidemia, chronic atrial fibrillation, severe mitral regurgitation (posterior leaflet prolapse) and moderate to severe tricuspid regurgitation, with pulmonary hypertension. She had undergone cardiothoracic surgery 15 days before, with mitral valve repair (quadrangular resection of the posterior leaflet with implantation of a Carpentier ring) and tricuspid annuloplasty. Postoperative transesophageal echocardiography (TEE) showed good mitral valve competence and no regurgitation, and no other significant alterations.
The patient was medicated with furosemide (40+20mg), enalapril 5mg once a day, carvedilol 6.25mg twice a day, amiodarone 200mg once a day, spironolactone 25mg once a day, potassium chloride (Retard) once a day, warfarin (for INR 2–3), omeprazole 20mg once a day and sertraline 50mg once a day.
On admission to the emergency department, the patient complained of precordial discomfort and worsening dyspnea on minimal exertion, as well as paroxysmal nocturnal orthopnea and dyspnea. Physical examination showed blood pressure of 86/64mmHg and mean heart rate (HR) of 150bpm; cardiac auscultation revealed arrhythmia and a grade III/VI systolic murmur over the aorta. Pulmonary auscultation revealed absence of breath sounds in the left lung base. There was no lower limb edema.
Laboratory tests showed normocytic and normochromic anemia (Hb 10.9g/dl) and worsening baseline renal function (urea 146mg/dl; creatinine 1.9mg/dl; creatinine clearance [by the MDRD formula] 27.69ml/min). The ECG revealed atrial fibrillation with mean ventricular response of 150bpm and poor R-wave progression in V1–V2. The chest X-ray showed cardiomegaly and moderate left pleural effusion.
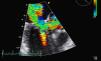
The patient was admitted for decompensated heart failure. Transthoracic echocardiography (TTE) performed on the first day of hospitalization (with HR 120–150bpm) (Figure 1) revealed aortic valve fibrosis with no restriction of opening, together with mild regurgitation.
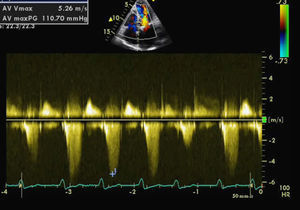
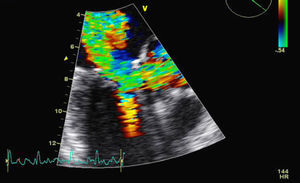
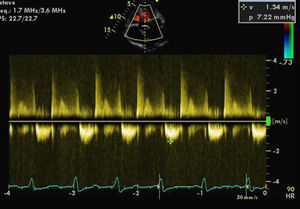
The MV presented fibrocalcification, with increased echogenicity of the annulus; the anterior leaflet and subvalvular apparatus were obstructing the LVOT, resulting in an intraventricular gradient of 110mmHg and moderate paroxysmal regurgitation (probably related to the intermittent nature of the LVOT obstruction). The left atrium was severely dilated (6.1cm), and the left ventricle was hypertrophied (diastolic diameter 4.3cm) but with good global systolic function. The right chambers were of normal size, with pulmonary artery pressure estimated at 40mmHg.
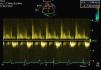
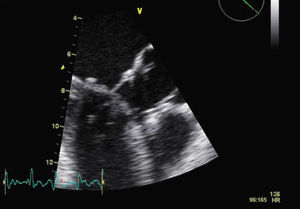
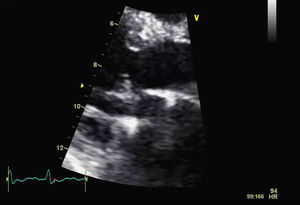
For a more accurate assessment of MV function, TEE was performed (with HR 120–150bpm) (Figures 2 and 3), which showed the MV with a Carpentier ring and leaflet degeneration and redundancy, good opening in diastole but with SAM leading to LVOT obstruction by the anterior leaflet, and severe regurgitation (vena contracta 8mm). The aortic valve was tricuspid, with good opening and mild regurgitation. The left atrial appendage was free of thrombi.
Since the patient's clinical condition was extremely unstable during hospital stay, systolic blood pressure remaining below 90mmHg and with clear signs of heart failure in NYHA class IV, the patient was transferred to a surgical center 14 days after admission to be evaluated for surgical reintervention.
Six weeks after her initial admission to our department, the patient was seen at the outpatient clinic; she was hemodynamically stable, in good general health and with no signs of heart failure. The report from the surgical center, where she had remained for three weeks, revealed that surgical reintervention had not been necessary. TTE at discharge showed significant improvement in echocardiographic parameters (mild mitral regurgitation and no LVOT obstruction by the mitral anterior leaflet). Repeat TTE a week after reassessment, with optimized HR, revealed good MV function (mild regurgitation), with no LVOT obstruction (Figures 4 and 5).
The literature indicates that SAM, which has been reported after mitral valve repair in various studies,3 is caused by the velocity of the blood flow drawing the ventricular surface of the MV anterior leaflet into the LVOT. The position and any abnormalities of the two leaflets contribute to the phenomenon.
Firstly, the distance between the MV coaptation point and the septum is shortened due to elongation of the posterior or anterior leaflets during surgical repair, increasing the area of the anterior leaflet exposed to LVOT flow.4
Secondly, during surgical repair of the papillary muscles, the MV may be displaced anteriorly around the LVOT, thus directly exposing the anterior leaflet to the outflow stream.5–7 Fluid overload in the pre- and postoperative period causes the septum to bulge leftwards and restrict the LVOT, while postoperative hypovolemia reduces left ventricular diastolic dimensions, thus decreasing LVOT diameter. All these pathophysiological conditions contribute to the development of SAM following mitral valve repair.1
However, there is some debate as to how and why the MV anterior leaflet is pushed towards the LVOT once the above-mentioned conditions are present.8–12 One theory is that it is due to a Venturi effect, the result of a fall in pressure distally to an obstruction. Pressure can be restored if there is dilatation distally to the stenosis with an angle of no more than 15°.4 The abrupt drop in pressure before the obstruction leads to the MV being sucked towards the LVOT. However, studies have measured the angle of MV leaflets at the point of coaptation and reported a mean of 21°, which goes against the above theory.13
Another mechanism proposed to explain this phenomenon is flow drag, which has been likened to an open door in a corridor subjected to strong gusts of wind. The stronger air flow in the middle of the corridor pushes the door in the direction of the air flow, exerting pressure on an increasing area of the door until it finally slams shut.3 Applying this analogy to the MV, it is possible that the flow drag of blood passing the anterior leaflet pulls it towards the LVOT and causes obstruction.
Other studies have proposed a combined mechanism, in which a Venturi effect lifts the leaflet towards the septum, while flow drag pulls the leaflet through it, closing the LVOT.14,15
Diagnosis of SAMEchocardiography, whether transesophageal or transthoracic, is essential to a diagnosis of SAM, as it reveals any residual parts of the MV that extend beyond the point of coaptation after valve repair and protrude into the middle of the LVOT, as well as showing a reduction in ventricular dimensions and/or septal bulging.1 In certain types of valve repair, the point of coaptation will be next to the septum. Patients with documented SAM can present with dyspnea, angina, palpitations, heart failure, syncope or arrhythmias, or a combination of symptoms.16
Management and treatment of SAM: is surgical reintervention necessary?There is uncertainty regarding the natural history and management of patients with SAM after MV repair. The degree of SAM extends along a spectrum from minor repercussions in the MV to its most severe form with LVOT obstruction.17 Despite numerous descriptions of preventative techniques, it continues to occur.18–21 Management of SAM by surgical means remains controversial, some groups advocating non-surgical treatment22,23 and others direct surgical correction.24–26
Both hypervolemia and hypovolemia can trigger SAM. Thus, a patient who has undergone MV repair may initially have normal TEE. Most patients undergoing cardiac surgery have hypovolemia, which plays a central role in the development of hyperdynamic SAM since the Venturi effect is more marked, resulting in decreased systolic volume and blood pressure.4 However, SAM can also occur in hypervolemic states. Increased right ventricular volume, which raises pulmonary pressure, causes the interventricular septum to bulge leftwards, narrowing the LVOT. In this situation, intravenous nitrates should be considered for immediate reduction of pulmonary pressure.27
Management of patients with SAM in the immediate postoperative period consists of keeping the left ventricle expanded to allow reasonable LVOT opening, for which crystalloid and colloid solutions are essential.1 Heart rate should be stabilized to maximize diastolic time. Tachyarrhythmias reduce ventricular filling time and affect end-diastolic volume and so beta-blockers are the first-choice drug in this context.13,16 Continuous infusion is recommended rather than a bolus, since the former is easier to titrate to reduce HR with the least effect on blood pressure.4
Positive inotropes such as epinephrine, which increase HR and contractility, should be used with caution in these patients2 since they have an adverse effect on left ventricular diastolic time, resulting in a hyperdynamic state and LVOT narrowing, thus increasing the severity of SAM. The overall aim of medical treatment is to maintain optimal left ventricular volume, which means that therapies that reduce peripheral vascular resistance should be avoided.4
Since SAM can be transient or persistent, treatment should be based on the severity of symptoms. If these are disabling or progressively worsen, surgical reintervention is recommended,4 the type of correction depending on the original surgery.28
Brown et al. carried out a major retrospective study of all patients between January 1993 and December 2002 in the Division of Cardiovascular Surgery of the Mayo Clinic in whom SAM occurred during the intraoperative period, and who were subsequently followed up.17 MV repair was performed in 2076 patients, in 174 (8.4%) of whom SAM was detected by intraoperative echocardiography. These patients were initially treated with a combination of beta-blockade, vasoconstriction with phenylephrine and/or intravascular volume expansion; four underwent surgical repair because of persistent SAM and three underwent late surgical reintervention because of mitral regurgitation from other causes. The median follow-up of the remaining 167 patients was 5.4 years. There were two other late reoperations, but neither was due to SAM or LVOT obstruction. Around 90% of patients were in NYHA class I, 7% in class II and 3% in class III or IV. Echocardiograms were available for review in 93 patients, of whom 13 had SAM and four had SAM with LVOT obstruction.
The above study's conclusions emphasized the fact that most cases of SAM were resolved with conservative treatment (beta-blockade, vasoconstriction and administration of fluids). Persistent SAM with LVOT obstruction was documented in 2.3% of patients but did not require late reintervention. The outcomes in this series (no mortality and 90% of patients in NYHA class I at late follow-up) support a strategy of non-surgical treatment of SAM, with or without LVOT obstruction.17
ConclusionsThe review of the literature carried out in order to answer the questions raised by our case led to the conclusion that late surgical reintervention is rarely required to treat SAM with LVOT obstruction following mitral valve repair, since it improves with optimized therapy and ventricular remodeling in the long term. Nevertheless, patients with SAM after MV repair need regular follow-up, beta-blockade and avoidance of afterload-reducing medications. Lifelong beta-blocker therapy is not generally required; if LVOT obstruction resolves, the dose can be titrated based on three-monthly echocardiographic study, and if no LVOT obstruction is detected, the patient can be reassessed at longer intervals.17
SAM is an important cause of mitral regurgitation early after MV repair, but optimized medical therapy can preclude the need for surgical reintervention.17
The phenomenon occurs with a variety of surgical techniques, and no ring or band, rigid or flexible, appears to have a direct influence on the outcome.17
To summarize, studies support a non-surgical approach to SAM, with or without LVOT obstruction.
Conflicts of interestThe authors declare they have no conflicts of interest.
Please cite this article as: Rodrigues, B. Obstrução severa do tracto de saída do ventrículo esquerdo como complicação de valvuloplastia mitral: a propósito de um caso clínico. Rev Port Cardiol. 2011;30(11):837–843.