Acute myocardial infarction (AMI) involving acute transmural ischemia of two vascular territories at the same time, which is known as double or combined infarction, is a well described phenomenon but rarely reported in most series of patients admitted for AMI. This may be related to the fact that AMI with multiple vessel obstruction often causes extensive myocardial injury and death before the patient arrives at the hospital. It is speculated that double infarction results from the overall prothrombotic and inflammatory conditions associated with AMI.
O enfarte agudo do miocárdio (EAM) envolvendo isquémia aguda transmural de dois territórios vasculares em simultâneo ou de forma sequencial é fenómeno bem descrito, que se designa por enfarte combinado ou duplo (ED,) mas habitualmente pouco relatado na maioria das séries de doentes admitidos por EAM. A sua rara apresentação está possivelmente relacionada com o facto de o EAM com obstrução de múltiplos vasos major causar lesão miocárdica extensa, impedindo a chegada do doente ao hospital com vida. Especula-se que o ED resulta das condições protrombóticas e inflamatórias globais associadas ao EAM.
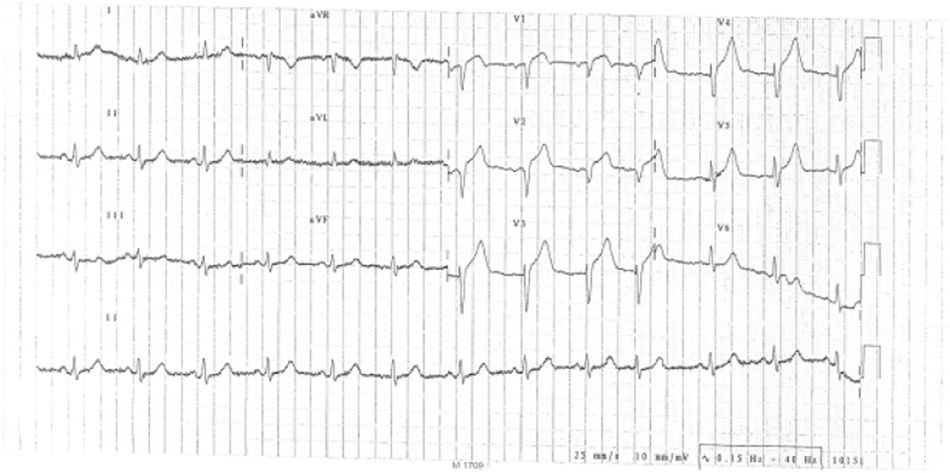
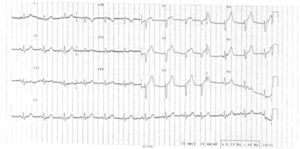
We report the case of a 78-year-old man, with no known vascular risk factors or comorbidities, who presented in the emergency department with constricting chest pain with onset at rest and of around two hours’ evolution. On admission, he was symptomatic, in Killip class I. The ECG showed sinus rhythm, with Q waves in V1 and V2 and ST elevation in V1–V5 (Figure 1). Since immediate percutaneous angioplasty was not available, he was treated by fibrinolysis with tenecteplase, together with standard medical therapy for ST-elevation acute coronary syndrome, and met clinical and ECG reperfusion criteria.
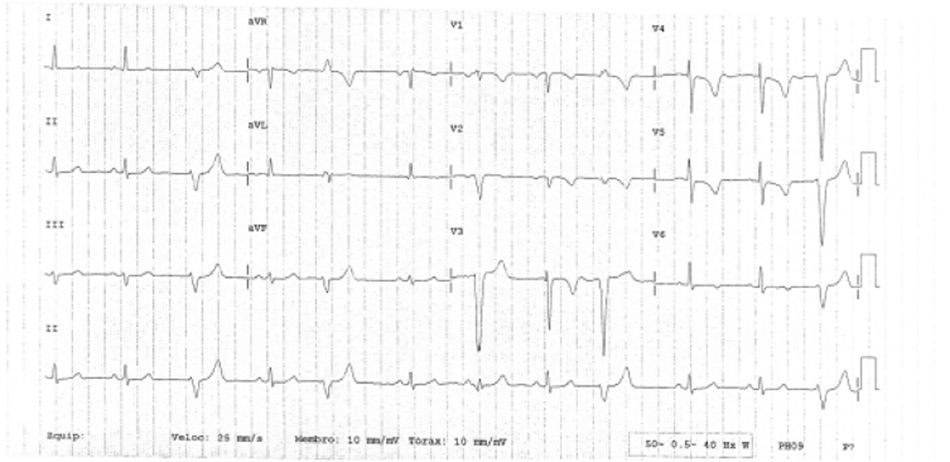
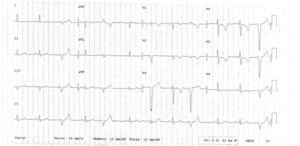
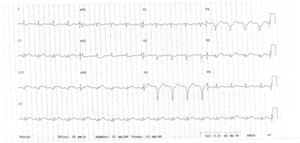
The clinical course was initially favorable, with no recurrence of pain, hemodynamic stability, Killip class I and T-wave inversion in V1–V6 (Figure 2). However, approximately 2h after fibrinolysis, the pain recurred, with hypotension and ECG alterations, notably ventricular tachycardia that degenerated to ventricular fibrillation. Immediate electrical defibrillation achieved reversion to sinus rhythm. ST elevation was observed in DII, DIII and aVF (Figure 3).
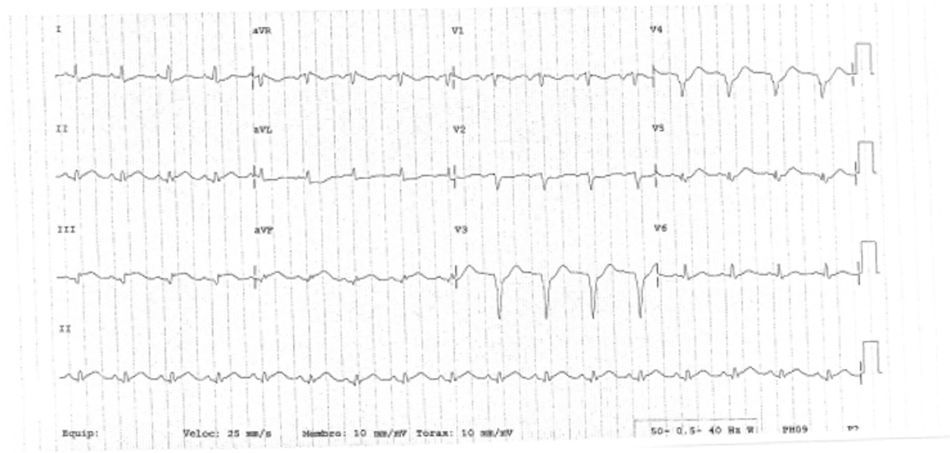
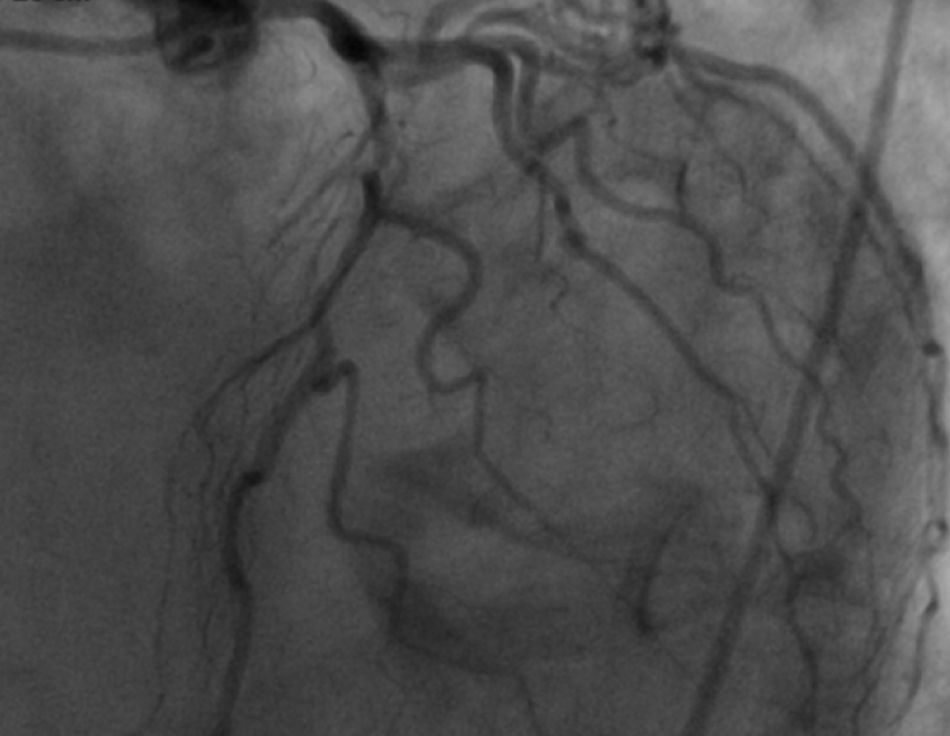
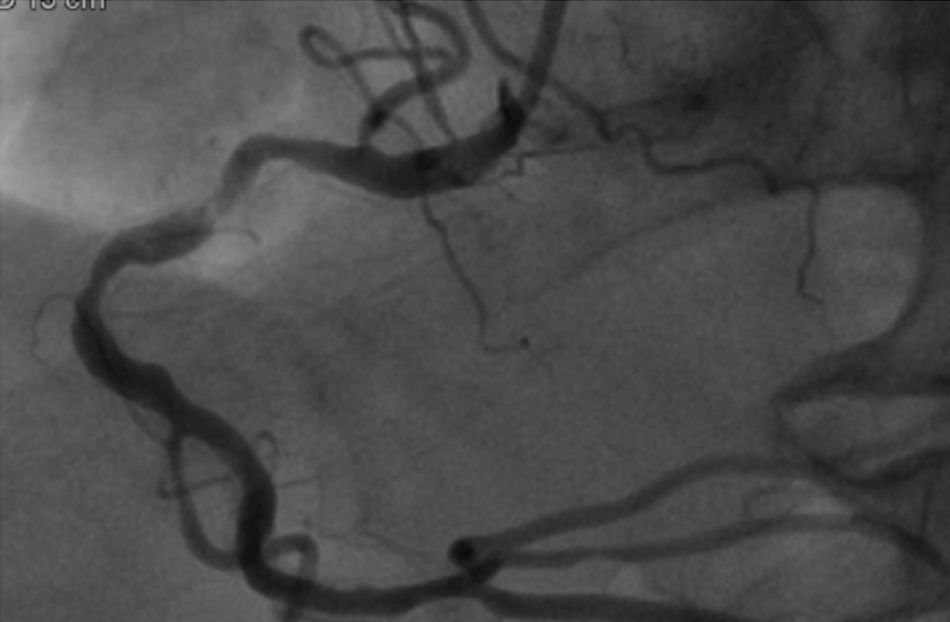
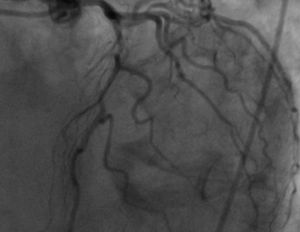
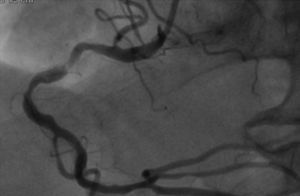
The patient was transferred to a center with hemodynamic laboratory for cardiac catheterization. On arrival, he was without pain and hemodynamically stable, but suffering from psychomotor agitation and spatial and temporal disorientation, in Killip class I; the ECG showed Q waves in the anteroinferior wall. Cardiac catheterization revealed three-vessel coronary disease, with subocclusive stenosis of the proximal segment of the left anterior descending artery (LAD) (culprit lesion), the proximal segment of the right coronary artery (RCA) (culprit lesion), and the distal segment of the circumflex artery (Cx). Angioplasty was performed with implantation of two Driver stents in the RCA and one in the LAD (Figures 4 and 5).
Following the procedure, the patient had no recurrence of angina, was electrically and hemodynamically stable in NYHA functional class II/IV, and recovered normal state of consciousness, in Killip class I, and with peak troponin I of 120.4ng/ml and BNP of 1661pg/ml. The pre-discharge echocardiogram showed mild to moderate depression of left ventricular systolic function with mean ejection fraction of 44%, akinesia of the anteroseptal wall and apex, and hypokinesia of the posteroinferior wall and distal segments of the lateral wall. He was transferred back to the original hospital, medicated with aspirin 100mg, clopidogrel 75mg, bisoprolol 2.5mg, lisinopril 20mg, rosuvastatin 10mg and long-acting isosorbide mononitrate 50mg, and with indication for elective percutaneous coronary intervention (PCI) on the Cx at a later date. One week after discharge, he was rehospitalized for ischemic stroke, with a partial infarction in the territory of the right middle cerebral artery which was not of cardioembolic or carotid etiology. Potential sources of hypercoagulability were investigated, including lupus anticoagulant, anticardiolipin antibodies, factor V Leiden, myeloproliferative disease and cancer, all of which were negative.
Discussion/ConclusionsAcute myocardial infarction (AMI) involving two or more culprit lesions at the same time, known as double or combined infarction, is a rare event with a poor prognosis.1 Most cases involve the right coronary and left anterior descending arteries,2 with one report of involvement of the left anterior descending and circumflex arteries.3 Although rarely reported in most series of patients admitted for AMI, autopsy studies reveal that thrombotic occlusion of more than one major epicardial coronary artery is not uncommon, occurring in up to 50% of patients with infarction.4 By contrast, Pollak et al. found 18 cases (2.5%) of multiple culprit arteries in a series of 711 patients undergoing primary PCI.5 This discrepancy is probably due to selection bias in that patients with multiple culprit arteries are more likely to suffer sudden cardiac death, not surviving long enough to undergo angiography. The results of Pollak et al.5 highlight the severity of the clinical symptoms associated with this condition, since a third of the patients presented in cardiogenic shock and a quarter had potentially fatal arrhythmias or required intra-aortic balloon pump. Although the factors involved in simultaneous acute thrombosis of multiple coronary arteries are not fully known, possible causes include: (1) heightened inflammatory response and catecholamine surge caused by the acute occlusion of one vessel, leading to a second coronary arterial occlusion; (2) hemodynamic instability and hypotension due to occlusion of one coronary artery, resulting in blood stasis and acute occlusion of another artery with a severe underlying lesion; (3) prolonged coronary vasospasm (due to Prinzmetal angina or cocaine use)6; (4) hypercoagulable states including malignancy,7 hyperhomocysteinemia8 and thrombocytosis9; and (5) coronary embolism.10 In the above study by Pollak et al., 19 (40%) out of 47 patients had comorbidities that were potential predisposing factors, including a history of cancer, HIV infection, cocaine use, coronary artery vasospasm, platelet abnormalities, atrial fibrillation and hyperhomocysteinemia, although in 60% of cases no contributing factors were identified.
The case presented here is unusual in that ischemia occurred sequentially, initially in the territory of the LAD and then in the RCA following antiplatelet, anticoagulant and fibrinolytic therapy, and that the patient suffered an ischemic stroke which was not of cardioembolic or carotid etiology two weeks after discharge under dual antiplatelet therapy, which would indicate an underlying hypercoagulable state as the likely cause of the clinical setting, despite negative tests. However, a heightened inflammatory response, as well as the hemodynamic instability and hypotension observed in a patient with severe underlying coronary disease, may also have contributed. Less likely, though possible, is coronary vasospasm or embolism.
We have presented a case of a single clinical setting involving two culprit lesions, which while rarely detected may be diagnosed more frequently in the future as AMI patients are treated earlier and more invasively.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ribeiro, H. Enfarte agudo do miocárdio duplo sequencial. doi 10.1016/j.repc.2011.10.001.