Cardiac remodeling is manifested as changes in size, shape and function of the heart. We studied the prevalence, prognosis and predictors of left ventricular reverse remodeling (LVRR) in idiopathic dilated cardiomyopathy (IDCM) after optimized medical therapy.
MethodsA total of 113 IDCM patients were followed for 7.1±5.6 years. LVRR was defined as an increase of 10 units in ejection fraction (EF) and decrease in left ventricular diastolic diameter (LVDD), in the absence of resynchronization therapy.
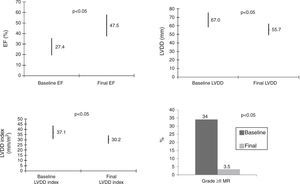
ResultsBaseline EF was 27±8% and LVDD index was 37.1±6.3 mm/m2. LVRR occurred in 34.5% within 22.6 months. Final EF was 47.5±10.1%, LVDD index was 30.2±3.9 mm/m2. LVRR was associated with better NYHA class (I–II) and lower BNP (p<0.01) and all patients were alive.
Univariate predictive factors of LVRR (p<0.05) were mild hypertension, atrial fibrillation, ventricular hypertrophy on ECG, absence of left bundle branch block, shorter QRS duration, higher hematocrit, lower LVDD index, higher peak oxygen uptake efficiency (VO2/log 10[VE]) and lower dVE/VCO2/VO2, treatment with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) and use of maximal doses of ACEI/ARB and beta-blockers. Multivariate regression analysis showed that higher doses of ACEI/ARB (OR: 0.32, 95% CI 0.11–0.92) were independently associated with LVRR. Non-transmural late enhancement on cardiac MRI was not a predictor of LVRR.
ConclusionsLVRR occurred in one third of IDCM patients, especially in those with mild hypertension and with less advanced disease, who may have benefited from maximal drug titration.
A remodelagem ventricular é caracterizada por alterações no tamanho, forma e função do coração. Estudámos a prevalência, o prognóstico e os fatores preditores de reversão da remodelagem do ventrículo esquerdo (RRVE) na miocardiopatia dilatada idiopática (MCDI), após a terapêutica farmacológica otimizada.
MétodosCento e treze doentes foram seguidos durante 7,1±5,6 anos. A RRVE foi definida como um aumento de dezunidades da fração de ejeção (FE) e diminuição do diâmetro diastólico do VE (VED), na ausência de terapêutica de ressincronização.
ResultadosA FE basal foi de 27±8% e o VED de 37,1 ± 6,3 mm/m2. A RRVE ocorreu em 34,5% dentro de 22,6 meses. A FE final foi de 47,5 ± 10,1%, o VED index foi de 30,2±3,9 mm/m2. A RRVE associou-se a melhor classe NYHA (I-II), menor BNP e a mortalidade nula.
Os preditores de RRVE foram hipertensão arterial (ligeira), fibrilhação auricular, hipertrofia ventricular esquerda (no ECG), ausência de bloqueio de ramo esquerdo, menor duração do QRS, maior hematócrito, menor VED index, melhor eficiência de oxigénio no pico do exercício (VO2/LG10[VE]), um menor DVE/VCO2/VO2, uso de IECA/ARA-II e uso de doses máximas de IECA/ARA-II e bloqueadores-β. Na análise multivariada o uso de doses máximas de IECA/ARA-II (OR: 0,32, 95% CI 0,11-0,92) foi um preditor independente. A presença ou extensão do realce tardio na RMN cardíaca não foi preditora de RRVE.
ConclusãoA RRVE ocorreu num terço dos pacientes MCDI, naqueles com hipertensão ligeira e com doença menos avançada, que poderão ter beneficiado da máxima titulação dos fármacos.
Cardiac remodeling is defined as genome expression resulting in molecular, cellular and interstitial changes and manifested clinically as changes in size, shape and function of the heart.1 The progression of heart failure (HF) is associated with left ventricular (LV) remodeling, which manifests as gradual increases in LV end-diastolic and end-systolic volumes, wall thinning, and a change in chamber geometry to a more spherical, less elongated shape, with a progressive decrease in ejection fraction (EF).
When ventricular remodeling is advanced, it begins to be self-sustaining and capable of driving disease progression, regardless of the patient's neurohormonal status. This may explain why medical therapies lose their effectiveness in end-stage HF, and why some device-based therapies (cardiac resynchronization and mechanical ventricular assistance), which can affect LV remodeling, are beneficial.
The overall importance of ventricular remodeling as a pathogenic mechanism and prognostic determinant is not clear. Some drug therapies and cardiac devices that increase the survival of patients with HF can slow, and in some cases even reverse, certain parameters of remodeling. Controversially, as in the case of etanercept2 and in cardiac resynchronization,3,4 reverse remodeling has not translated into increased survival. Additionally, the molecular mechanisms of reverse remodeling have not been fully elucidated.
Left ventricular reverse remodeling (LVRR) is characterized by a decrease in LV dimensions, normalization of LV shape and improvement of systolic function.
A significant prevalence of recovery of LV function in patients with dilated cardiomyopathy (DCM) has been reported.5 However, such studies included patients with new-onset DCM like acute myocarditis, and other reversible causes of DCM, such as peripartum and alcohol-related DCM. The mechanisms underlying LVRR in such situations appear to be different from those involved in chronic idiopathic DCM.
The aim of this prospective study was to assess recovery of LV function and reversal of ventricular remodeling in patients with chronic idiopathic DCM, after optimized medical therapy. We set out to assess its prevalence, to identify its predictors and to determine whether it was associated with better prognosis.
MethodsThe study included consecutive adult patients with idiopathic DCM (left ventricular diastolic diameter [LVDD] >33 mm/m2 in men, >32 mm/m2 in women) between 2000 and June 2012 followed in an HF clinic, diagnosed less than 24 months previously and with two initial values of left ventricular ejection fraction (LVEF) of <0.40 more than one year apart.
We excluded DCM patients with secondary etiologies, including a history of myocardial infarction or angina, those with ischemia or significant coronary disease on coronary angiography, a history of moderate or severe hypertension, at least moderate primary mitral or aortic valvular disease, heavy alcohol use (>100 g/day), chemotherapy-induced and peripartum cardiomyopathy, acute HF with biopsy positive for acute myocarditis or positive serology for acute bacterial or viral infection. We included patients with idiopathic DCM, diagnosed after respiratory infections but with LV dysfunction that persisted for over a year (in order to exclude myocarditis). We also excluded patients with uncontrolled atrial and ventricular arrhythmias.
At baseline, patients underwent clinical assessment, electrocardiogram (ECG), 24-hour ECG, transthoracic echocardiogram, blood laboratory measurements, cardiopulmonary exercise testing (CPET) and cardiac magnetic resonance (CMR).
Patients were managed according to current clinical practice guidelines and clinicians aimed to reach the recommended target doses for all therapies.
During follow-up, periodic clinical assessment, laboratory measurements and echocardiogram were performed.
This study was in accordance with the recommendations set by the Declaration of Helsinki and with local legal requirements.
Definition of left ventricular reverse remodelingLVRR was defined as an absolute increase on two consecutive echocardiograms more than six months apart of 10 units of LVEF, together with a decrease in left ventricular diastolic diameter (LVDD), without worsening of mitral regurgitation, in the absence of cardiac resynchronization therapy (CRT) or mechanical ventricular assistance.
Transthoracic echocardiography protocolTransthoracic echocardiography was performed at baseline and during follow-up using two commercially available systems: General Electric Vivid 3.0 and Vivid 7.0 with a 2.5-MHz transducer. The following parameters were measured according to the standards defined by the American Society of Echocardiography and the European Association of Echocardiography6: LVDD and end-systolic diameter; LV EF (%) calculated by Simpson's biplane method; degree of mitral regurgitation by Doppler and color Doppler, on a scale from 0 to 4; left atrial diameter; LV posterior wall thickness and interventricular septal thickness; right ventricular systolic dysfunction (defined as tricuspid annular systolic excursion [TAPSE] <16 mm); and pulmonary artery systolic pressure (PASP) calculated by tricuspid velocities. Data on diastolic function were incomplete.
Patients who received CRT were considered have no LVRR, so EF and LVDD before CRT were included in the analysis.
All data were digitally stored, and off-line data analysis was performed by two echocardiography specialists, blinded to the study.
Cardiopulmonary stress testingPatients underwent maximal symptom-limited CPET (Jaeger Oxycon Mobile 4.6). Blood pressure was measured manually and a modified Bruce protocol was used. All tests were interrupted due to symptoms. Expired ventilatory flow (VE), oxygen uptake (VO2), carbon dioxide output (VCO2) and other cardiopulmonary variables were acquired breath-by-breath by pneumotachograph with bidirectional differential pressure. Peak oxygen uptake (VO2 peak) was calculated as the mean values during the last 30 s of effort. The anaerobic threshold (AT) was calculated automatically by the V-slope method. We also determined circulatory power (VO2 peak×peak systolic blood pressure), VE/VCO2 slope, ventilatory equivalent for oxygen (VE/VO2) and VE/CO2 slope normalized for peak VO2. Because of the limitations of the system, instead of calculating the oxygen uptake efficiency slope, we calculated peak oxygen uptake efficiency (POUE) (peak VO2/log 10 peak VE) at AT, which is more easily obtained and has similar prognostic value.7
The Heart Failure Survival Score (HFSS) was calculated by the equation: (0.0216×heart rate)+(−0.0255×mean blood pressure)+(−0.0464×EF)+(−0.0470×Na+ concentration)+(−0.0546×peak VO2)+(0.6083×QRS>120 ms 1, no 0)+(0.6931×ischemic etiology 1, no 0).
Cardiac magnetic resonanceThe CMR studies were performed on a 3 T clinical scanner (Siemens® Magnetom Trio). Electrocardiogram-gated cine steady-state free precession imaging was performed in short-axis and orthogonal LV long-axis views. A breath-hold, T2-weighted dark blood sequence was acquired. Late gadolinium enhancement (LGE) images were acquired 10–15 min after gadolinium administration using a phase-sensitive inversion-recovery sequence.
The extent of LGE was quantified by the number of segments affected. The presence and distribution of LGE were independently determined by one radiologist and one cardiologist, blinded to the study.
Statistical analysisAll values are reported as mean ± SD, median ± interquartile range or percentages according to data characteristics. Differences between subjects in each arm were assessed using the chi-square test for categorical variables and the Student's t test or the Mann-Whitney test for continuous variables, as appropriate. A two-tailed p<0.05 was considered to indicate statistical significance.
To assess predictors of LVRR from baseline characteristics and from therapy, univariate analysis included all relevant clinical or laboratory parameters. Variables with p<0.05 from the univariate analysis were entered in multivariate Cox regression analysis, but variables with low quantities of data (those from 24-hour ECG, CPET and CMR) were excluded.
ResultsPopulation characteristicsA total of 113 patients were included, followed for 7.1±5.6 years, mean age 50±14 years; 74 were male (66%).
At baseline, mean EF was 27±8%, LVDD was 67±9 mm, LVDD index was 37.1±6.3 mm/m2 and grade >II mitral regurgitation was present in 34% of patients.
On ECG, 44% had left bundle branch block (LBBB), 46% had LV conduction disturbances and 14% had atrial fibrillation. The majority of patients were in NYHA class II (69%). Table 1 details the patients’ baseline clinical characteristics.
Baseline characteristics of the study population at baseline (n=113).
| Age (years) | 50.1±14.5 |
| Male (%) | 65.5 |
| Body mass index | 27.1±3.9 |
| Hypertension (%) | 39.8 |
| Diabetes (%) | 17.7 |
| Chronic pulmonary disease (%) | 8.8 |
| Moderate alcohol intake | 21.2 |
| Heart rate (bpm) | 80.2±17.7 |
| Systolic blood pressure (mmHg) | 119±20 |
| NYHA class I (%) | 20.4 |
| NYHA class II (%) | 69.0 |
| NYHA class III (%) | 8.8 |
| NYHA class IV (%) | 1.8 |
| Atrial fibrillation (%) | 14.2 |
| LBBB (%) | 44.2 |
| QRS duration (ms) | 126.6±31.9 |
| LV hypertrophy (%) | 21.2 |
| Echocardiography | |
| RV dilation (%) | 11.5 |
| RV dysfunction (%) | 8.0 |
| TAPSE (mm) | 23.4±4.5 |
| Grade >II/IV tricuspid regurgitation | 5.4 |
| PASP (mmHg) | 39.6±16.0 |
| LVDD (mm) | 67.0±8.7 |
| LVSD (mm) | 57.0±8.1 |
| LVDD/BSA (mm/mm2) | 37.1±6.4 |
| Ejection fraction (%) | 27.2±8.2 |
| LV mass/BSA (g/m2) | 185.1±30.4 |
| Grade >II/IV mitral regurgitation (%) | 33.6 |
| Left atrial diameter (mm) | 45.7±6.6 |
BSA: body surface area; LBBB: left bundle branch block; LV: left ventricular; LVDD: left ventricular end-diastolic diameter; LVSD: left ventricular end-systolic diameter; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; RV: right ventricular; TAPSE: tricuspid annular systolic excursion.
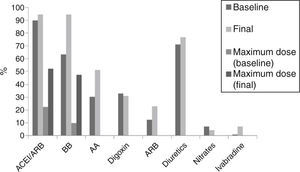
At the end of follow-up, 90% were treated with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), 64% with beta-blockers, 30% with aldosterone antagonists and 33% with digoxin. Optimal recommended doses of ACEI/ARB were reached in 52.2% (20–30 mg lisinopril, 5–10 mg perindopril, 16–32 mg candesartan) and optimal doses of beta-blockers were reached in 47.8% (25–50 mg bid carvedilol, 5–10 mg bisoprolol). Figure 1 shows therapy at baseline and at the end of follow-up.
Urgent heart transplantation or death occurred in 16% of patients (nine deaths, nine transplantations), 38% were hospitalized for worsening HF and 30% had cardiac devices implanted: implantable cardioverter-defibrillator (ICD) in 19%, CRT plus ICD in 8%, and CRT pacing in 3%.
Prevalence and prognostic value of left ventricular reverse remodelingInitial EF in patients who recovered LV function was 28±9%, not significantly different from the 27±9% in those who did not recover.
LVRR occurred in 39 patients (34.5%) within 22.6 months (median). Final EF was 47.5±10.1% (Δ EF 19.4±9.0%), LVDD was 55.7±6.7 mm (Δ LVDD −9.6±−7.4 mm), LVDD index was 30.2±3.9 mm/m2 and only 3.5% had grade >II MR (Figure 2).
Patients with LVRR had better NYHA functional capacity: class I (67% vs. 25%, p<0.01), class II (43% vs. 31%, p<0.01) and had lower BNP (median 27.4 vs. 160.0 pg/ml, p<0.01), compared with those without LVRR. LVRR was associated with lower rates of HF hospitalization (23.1% vs. 44.6%, p=0.02), cardiac death and urgent transplantation (0.0% vs. 24.3%, p<0.01).
Factors predicting left ventricular reverse remodelingBecause of technical reasons and pre-existing contraindications, only 89 patients underwent 24-hour ECG, only 55 patients underwent CPET and only 38 underwent CMR at baseline.
Variables at baseline that predicted LVRR were (Table 2): mild hypertension (54% vs. 32%, p<0.05), atrial fibrillation (26% vs. 8%, p<0.05), ventricular hypertrophy on ECG (36% vs. 14%, p<0.05), absence of LBBB (31% vs. 51%, p<0.04), shorter QRS interval (117 ms vs. 131 ms, p<0.05), higher hematocrit (43.2 vs. 40.8%, p<0.05), lower LVDD index (35.4 vs. 38.0 mm/m2, p<0.05) and less non-sustained ventricular tachycardia on 24-hour ECG (12.5% vs. 33.9%, p=0.03).
Baseline variables predicting left ventricular reverse remodeling.
| LVRR (n=74) | No LVRR (n=39) | p | OR | CI | |
|---|---|---|---|---|---|
| Age (years) | 49.8±14.5 | 49.2±13.9 | 0.84 | ||
| Male (%) | 64.9 | 66.7 | 0.85 | ||
| Hypertension (%) | 32.4 | 53.8 | 0.03 | 2.4 | 1.1–5.4 |
| NYHA class I (%) | 21.6 | 17.6 | 0.91 | ||
| Heart rate (bpm) | 78.6±16.9 | 83.3±19.1 | 0.18 | ||
| Systolic blood pressure (mmHg) | 117.0±20.7 | 122.7±18.9 | 0.16 | ||
| Atrial fibrillation (%) | 8.1 | 25.6 | 0.01 | 3.9 | 1.3–11.8 |
| QRS duration (ms) | 131.8±32.2 | 117.1±29.4 | 0.02 | 0.9 | 0.9–0.98 |
| LBBB (%) | 51.4 | 30.8 | 0.03 | 0.4 | 0.2–0.9 |
| LV hypertrophy (%) | 13.5 | 35.9 | 0.01 | 3.5 | 1.4–9.0 |
| Laboratory variables | |||||
| Hematocrit (%) | 40.8±4.0 | 43.2±3.1 | 0.01 | 1.2 | 1.1–1.3 |
| Creatinine clearance (ml/min) | 99.7±32.9 | 107.1±29.1 | 0.24 | ||
| Uric acid (mg/dl) | 35.7±30.9 | 40.4±31.1 | 0.55 | ||
| Na+ (mEq/l) | 138.6±2.8 | 139.7±2.6 | 0.06 | ||
| BNP (pg/ml) (median) | 65.0±204.8 | 26.2±1839.0 | 0.48 | ||
| Echocardiogram | |||||
| LV ejection fraction (%) | 27.0±9.0 | 28.1±8.7 | 0.46 | ||
| RV dysfunction (%) | 8.1 | 7.9 | 0.97 | ||
| LA diameter (mm) | 46.1±7.2 | 44.8±5.2 | 0.30 | ||
| LA volume/BSA (ml/m2) | 38.1±16.0 | 37.6±11.3 | 0.93 | ||
| LV diameter (mm) | 68.0±9.5 | 65.1±6.8 | 0.09 | ||
| LV diameter/BSA (mm/m2) | 38.0±7.0 | 35.4±4.5 | 0.04 | 0.9 | 0.86–0.99 |
| LV mass/BSA (g/m2) | 337.9±109.2 | 315.8±71.1 | 0.26 | ||
| Grade >II/IV mitral regurgitation (%) | 36.5 | 28.2 | 0.37 | ||
| PASP (mmHg) | 39.9±17.1 | 39.1±13.3 | 0.86 | ||
| 24-hour ECG | n=57 | n=32 | |||
| Mean HR (24 hour ECG) (bpm) | 74.2±9.6 | 78.1±11.5 | 0.10 | ||
| Non-sustained VT (%) | 33.9 | 12.5 | 0.03 | 0.3 | 0.1–0.9 |
| SDNN (ms) | 101.7±38.1 | 125.7±53.3 | 0.09 | ||
BNP: natriuretic brain peptide; CI: confidence interval; HR: heart rate; LA: left atrial; LV: left ventricular; LVVR: left ventricular reverse remodeling; OR: odds ratio; SDNN: standard deviation of NN interval; VT: ventricular tachycardia. Other abbreviations as in Table 1.
Predictor variables from CPET were higher POUE (0.879 vs. 0.734, p<0.05) and lower dVE/VCO2/VO2 (2.5 vs. 4.0, p<0.05) (Table 3).
Predictive factors of left ventricular reverse remodeling on cardiopulmonary exercise testing and cardiac magnetic resonance imaging.
| No LVRR | LVRR | p | OR | CI | |
|---|---|---|---|---|---|
| CPET | n=41 | n=14 | |||
| Peak VO2 (ml/kg/min) | 17.6±5.6 | 19.9±4.9 | 0.16 | ||
| %VO2 predicted (%) | 59.8±17.8 | 68.4±18.8 | 0.13 | ||
| % VO2 at AT (%) | 39.1±17.1 | 43.8±13.3 | 0.38 | ||
| VE/CO2 slope | 40.9±14.7 | 35.3±7.8 | 0.20 | ||
| VE/VCO2/VO2 peak | 4.0±3.4 | 2.5±1.4 | 0.05 | 0.7 | 0.4–1.0 |
| O2 pulse (%) | 78.9±26.6 | 84.9±23.0 | 0.46 | ||
| Circulatory power (mmHg/ml/kg/min) | 2415.9±866.3 | 2893.5±914.0 | 0.09 | ||
| POUE | 734.0±245.2 | 979.0±181.6 | 0.03 | 1.01 | 1.0–1.1 |
| POUE at AT | 274.9±17.1 | 327.2±80.9 | 0.09 | ||
| Δ HR recovery at 1 min (bpm) | 18.4±8.2 | 22.9±9.8 | 0.22 | ||
| CMR | n=24 | n=14 | |||
| EF (%) | 30.4±10.1 | 34.4±9.0 | 0.24 | ||
| Cardiac index (l/min/mm2) | 3.1±0.7 | 2.9±0.3 | 0.53 | ||
| RV EF (%) | 47.9±1.1 | 52.3±7.8 | 0.23 | ||
| LGE (%) | 58.3 | 50.0 | 0.74 | ||
| LGE >one segment (%) | 50.0 | 42.9 | 1.0 | ||
AT: anaerobic threshold; CI: confidence interval; CMR: cardiac magnetic resonance; CPET: cardiopulmonary exercise testing; EF: ejection fraction; LGE: late gadolinium enhancement; LV: left ventricular; LVRR: left ventricular reverse remodeling; OR: odds ratio; POUE: peak oxygen uptake efficiency; RV: right ventricular; VCO2: carbon dioxide output; VE: expired ventilatory flow; VO2: oxygen uptake.
Mean calculated HFSS was 8.97±0.85, with 98.2% of patients at low risk and only 1.8% at medium risk, and did not differ in patients who did not recover EF.
Non-transmural LGE (showing midwall fibrosis) on CMR was present in 55.3% of patients; in 26.3% it was limited to one LV segment and in 28.9% it was observed in more than one segment. LGE or other CMR parameters, such as right ventricular EF, were not predictors of LVRR (Table 3).
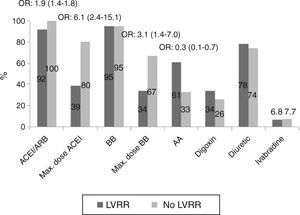
During follow-up, patients in the LVRR group were more often treated with ACEI/ARB (100% vs. 92%, p<0.05) and with maximal doses (80% vs. 39%, p<0.01). There were no differences in the use of beta-blockers, but those who had LVRR more often reached maximal doses (67% vs. 34%, p<0.01) and were less often medicated with aldosterone antagonists (33% vs. 61%, p<0.01) (Figure 3).
Pharmacological predictors of reverse remodeling during follow-up, showing differences in percentages of medical therapy between patients with and without left ventricular reverse remodeling. AA: aldosterone antagonists; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; BB: beta-blockers; LVRR: left ventricular reverse remodeling; Max.: Maximum; OR: odds ratio.
Multivariate regression analysis showed that only treatment with recommended doses of ACEI/ARB (OR: 0.32, 95% CI 0.11–0.92) was independently associated with LVRR.
DiscussionIn the present study, we describe the frequency of improvement in LV systolic function in patients with chronic idiopathic DCM in an unselected population.
LVRR has been described in secondary forms of DCM, such as peripartum cardiomyopathy, alcohol abuse, myocarditis and ischemic heart disease, but the mechanisms underlying such conditions are different from those in idiopathic DCM.8,9
A significant prevalence of recovery of LV function has also been described in recent-onset DCM. Those patients have a higher potential for LVRR, due to resolution of the underlying disease, as in myocarditis, or to favorable effects of therapy. Kubanek et al.10 reported a prevalence of 45% of LVRR at 12 months in 44 patients with recent-onset DCM, including some with active and resolving myocarditis. We only included patients with idiopathic DCM diagnosed less than 24 months previously, but with two initial values of EF of <0.40 more than one year apart, in order to exclude resolving myocarditis.
In our population, LVRR occurred in approximately one third of patients within 22 months of diagnosis. It was associated with improvement in NYHA functional class, with decrease in BNP compared with those who did not recover, and with excellent prognosis.
Recovery in EF and reverse remodeling was associated with maximal treatment with ACEI/ARB and beta-blockers. Patients with LVRR were less often medicated with aldosterone antagonists, probably because they achieved better NYHA functional class.
A favorable response to drug therapy with ACEI, beta-blockers and aldosterone antagonists was reported, with almost complete reversal of LV dysfunction. An increase in EF of more than 15 units has been described, associated with increases in functional capacity and cardiac index and a decrease in pulmonary capillary pressure, associated with a better prognosis.11–14 Treatment of HF can influence hemodynamics by decreasing LV afterload and preload. The experimental literature suggests that alterations in the biology and contractility of the failing cardiac myocyte may be reversible after beta blockade. Recent studies in patients treated with beta-blockers who had an increase in EF also showed favorable changes in myocardial gene expression: an increase in sarcoplasmic reticulum calcium ATPase mRNA and alpha-myosin heavy chain mRNA and a decrease in beta-myosin heavy chain mRNA.15
In our study, patients with LVRR more often had hypertension and appeared to be at an earlier stage of the disease, with lower LVDD, shorter QRS interval, less LBBB and more favorable ventilatory efficiency. Patients with hypertension and LV dysfunction respond to appropriate afterload-reducing therapy with improvements in LV function, and probably more frequently and more rapidly reach maximum drug titration with beta-blockers and ACEI.
Although only 14% of patients had AF at first consultation, the higher percentage of AF among patients who recovered EF was somewhat surprising. One possible explanation is that AF might have developed simultaneously with heart failure, causing functional changes (irregular and rapid rhythm, loss of atrioventricular synchrony, and loss of atrial transport), which would then show maximum benefit from medical therapy, with reversal of ventricular dysfunction.16
The predictors of RRVE in CPET were higher POUE and lower dVE/VCO2/VO2. Decreased oxygen efficiency slope and lower ventilatory efficiency, determined by the VE/CO2 slope, additionally normalized for peak VO2, are sensitive and early prognostic factors of heart failure, reflecting more advanced disease.17,18
Our results are consistent with other studies that set out to define the clinical variables associated with improvement in LVEF. Cicoira et al.19 evaluated 98 patients with idiopathic DCM, and found that those who recovered LV systolic function had shorter duration of symptoms, worse NYHA class and a history of hypertension. In a large study,20 LVRR was found in 89 of 242 idiopathic DCM patients (37%) and baseline predictors were higher systolic blood pressure and absence of LBBB. Binkley et al.21 showed that patients who recovered LV function were younger, had higher systolic blood pressure, lower serum creatinine, shorter QRS interval, lower prevalence of diabetes and higher prevalence of hypertension, were more frequently female and had a lower prevalence of ischemic cardiomyopathy.
It has been postulated that non-ischemic etiology has a higher probability of reverse remodeling. This appears to be related to a higher degree of adrenergic activation for an equivalent degree of myocardial dysfunction and to a greater extent of viable myocardium in patients with idiopathic DCM. A marked reduction in sympathetic activity appears to reduce mortality. The extent of heart rate reduction, rather than its baseline level, appears to be associated with a greater increase in LV function.22
Contractile reserve has been suggested as a key predictor of LVRR, according to studies with dobutamine echocardiography23 and positron emission tomography.24 CMR with gadolinium administration indirectly demonstrates contractile reserve in patients with idiopathic DCM, through the presence of myocardial fibrosis. Some studies have assessed the prognostic value of CMR in non-ischemic DCM. In the study by Assomull et al., 25 midwall fibrosis was present in 35% of 101 patients and was associated with a higher rate of the primary combined endpoint of all-cause death and cardiovascular events. In one study of recent-onset DCM, the lower extent of LGE and the higher edema ratio at CMR were the most important baseline predictors of LVRR.10 In our study, the presence or extent of LGE was not a predictor of LVRR, possibly due to the small study population.
QRS duration is one of the most sensitive independent predictors of survival in patients with DCM. In our population, mean QRS duration of patients who did not recover LV function was 130 ms. This finding is consistent with recommendations for biventricular pacing. Patients with LVRR also less often had non-sustained ventricular tachycardia on 24-hour ECG, probably also reflecting some positive electrical remodeling.
To summarize, these variables probably discriminate patients in whom EF can recover with medical therapy only from those who may require resynchronization devices or more aggressive strategies, including heart transplantation. Patients whose LV function recovers no longer have indication for ICD or CRT therapy, thus complicating the timing of implantation of these devices. Although current guidelines suggest that an ICD is indicated only in patients already receiving maximal medical therapy, it is not clear how safe it is to wait for optimization of therapy before ICD implantation. We can postulate that in patients with LBBB, low systolic blood pressure and larger LV diameters, it may not be safe to wait for ICD/CRT implantation.
Study limitationsIn this study we did not perform the expected number of CMR and CPET exams.
Another study is ongoing in our HF clinic, in a cohort of idiopathic DCM patients, all in sinus rhythm, assessing emerging laboratory predictors of LVRR and obtaining detailed echocardiographic data, with volumetric measures and myocardial deformation changes.
ConclusionsLVRR occurred in approximately one third of patients with idiopathic DCM, and these patients appeared to be at an early stage of the disease, had higher blood pressure and had maximal therapy titration. In these cases there is no longer indication for ICD or CRT implantation, thus complicating the timing of implantation of these devices.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.