Endothelial progenitor cells (EPCs) have an important role in vascular repair. Levels in peripheral circulation are thought to be related to overall cardiovascular risk and may represent potential therapeutic targets. The aim of this work is to identify predictors of circulating EPC concentrations in patients without known coronary artery disease (CAD).
MethodsThe study population consisted of 215 patients without known CAD referred for multidetector computed tomography (MDCT) coronary angiography (CTA) during a 6-month period. All patients underwent: 1) short anamnesis; 2) anthropometric measurements; 3) blood pressure and heart rate assessment; 4) blood tests; and 5) MDCT (including quantification of visceral fat, quantification of coronary artery calcification [CAC] and CTA).
ResultsThe patients’ mean age was 58±11 years (26-84) and 61% were male. Dyslipidemia (59%) and hypertension (57%) were the most prevalent risk factors. Twenty-seven percent met the ATP III criteria for metabolic syndrome. Mean Framingham risk score was 12±9%. Sixty-seven percent had no significant CAD but 64% had some degree of coronary calcification. The mean CAC (Agatston) was 186±433.
Mean EPC concentration, expressed as a percentage of total white blood cells, was 0.05±0.08% (0.0-0.58%). EPCs were inversely related to the presence of diabetes mellitus and smoking, and positively related to C-reactive protein. No significant correlations were found between EPCs and other risk factors, measurements of adiposity, atherosclerotic burden or severity of CAD.
ConclusionIn patients without known CAD referred for MDCT, EPC levels in peripheral blood cannot be significantly estimated or predicted from knowledge of patient anamnesis, risk factors, visceral fat, CAC or CTA.
As células progenitoras endoteliais (EPC) desempenham um papel primordial no processo de reparação vascular. Os seus níveis circulantes no sangue periférico parecem estar relacionados com o risco cardiovascular global e podem representar potenciais alvos terapêuticos. O objectivo deste estudo foi identificar eventuais preditores da concentração de EPC em pacientes sem doença coronária (CAD) conhecida.
População e métodosForam incluídos 215 doentes sem CAD conhecida, referenciados para coronariografia por tomografia computadorizada multidetectores (MDCT), durante um período de 6 meses. Todos os doentes foram submetidos a: 1) anamnese, 2) medidas antropométricas, 3) avaliação da pressão arterial e frequência cardíaca, 4) estudo analítico e 5) MDCT (incluindo a quantificação da gordura visceral, quantificação da calcificação coronária (CAC) e angiografia coronária).
ResultadosA média de idades era de 58±11 anos (26-84), 61% do sexo masculino. Os factores de risco vascular mais frequentes foram dislipidemia (59%) e hipertensão (57%). Vinte e sete por cento dos doentes cumpriam os critérios ATP III para a síndrome metabólica. O Framingham Risk Score médio foi de 12±9%. Sessenta e sete por cento não tinham CAD significativa, mas 64% apresentavam algum grau de calcificação coronária. A CAC média (Agatston) foi de 186±433. A concentração média de EPC, expressa em percentagem do total de leucócitos, foi de 0,05±0,08% (0,0-0,58%). A concentração de EPC correlacionou-se inversamente com a presença de diabetes mellitus e tabagismo e positivamente com a Proteína C Reactiva. Não houve correlações significativas entre os níveis de EPC e outros factores de risco, medidas de adiposidade, carga aterosclerótica total ou gravidade da CAD.
ConclusãoEm pacientes sem CAD conhecida referenciados para MDCT, os níveis de EPC no sangue periférico não podem ser estimados a partir do conhecimento do contexto clínico, factores de risco, gordura visceral, CAC ou coronariografia.
Vascular endothelium plays a pivotal role in cardiovascular biology. It is continually renewed and repaired in response to aggressions, in a process called angiogenesis. Various circulating cells are involved in this regeneration process: mature endothelial cells, monocytes capable of phenotypic and functional differentiation into endothelial cells (CD14+), and endothelial progenitor cells (EPCs). EPCs are derived from pluripotent bone marrow cells and, despite some disagreement about their phenotypic characterization, can be recognized in peripheral blood samples according to membrane receptor expression. Three different groups of EPCs in peripheral blood are usually considered: CD34+/133+, CD34+/133+/KDR+ and CD34+/KDR+ cells. The latter group is the most differentiated and represents about 80% of circulating EPCs1. Cells with CD34 and KDR membrane receptors — which can be detected using immunocytochemical techniques — have become one of the most common areas of study in angiogenesis research2.
EPCs bind to sites of vascular injury, promoting regeneration of endothelial tissue. They have been described as potential markers of cardiovascular risk and as potential therapeutic targets for cardiovascular disease. Lower levels of EPCs in peripheral blood have been associated with several cardiovascular risk factors and with coronary artery disease (CAD)3-7. However, little is known about their relationship with indirect risk factors such as visceral obesity8. Furthermore, their correlation with atherosclerotic burden in subjects without known CAD is not well documented9.
Before EPC levels can be considered for risk assessment and before therapies can be planned to take advantage of their vascular repair properties, circulating EPC levels need to be studied in different populations and in different clinical situations10,11. Study of the possible correlation of circulating EPC levels with demographic parameters, anthropometric measures and traditional risk factors may contribute to understanding of the molecular mechanisms involved in the atherosclerotic disease and may play a role in the identification and selection of candidates for therapies based on the therapeutic potential of angiogenesis.
The aim of this work was to identify predictors of circulating EPC levels in peripheral blood and to study their correlation with direct and indirect cardiovascular risk factors in patients referred for CT angiography.
MethodsSubjectsBetween November 2007 and May 2008, 261 consecutive patients referred for multidetector computed tomography (MDCT) for non-invasive assessment of CAD due to symptoms suggestive of ischemia were prospectively screened for study eligibility in a Portuguese hospital (Centro Hospitalar de Vila Nova de Gaia). Patients with known CAD (n=16), patients requiring different protocols for MDCT imaging, such as simultaneous request for aorta evaluation or combined assessment of coronary, aorta and pulmonary arteries (“triple rule-out”) (n=7), patients with very unstable rhythms (n=5), known anemia (Hb ≤8.5) (n=2), known iodine-based contrast allergy (n=1) and renal insufficiency (n=2), or with severe mobility limitations that could preclude accurate anthropometric measurements (n=5), and patients who refused or were unable to give written informed consent (n=8), were excluded. The final population consisted of 215 symptomatic patients (132 male, 83 female) without known CAD. Written informed consent was obtained from all participants.
Study designAll the 215 subjects in the final study population underwent the same protocol.
Assessment of clinical history and known risk factorsAfter written informed consent was obtained, a brief clinical interview was performed and clinical information (including cause of referral, symptoms, previous clinical history, known risk factors, previous examinations and medication) was obtained using a standardized health questionnaire and referral letters. Hypertension was defined as a history of known elevated blood pressure diagnosed and treated with medication, diet, and/or exercise for at least one year or requirement for antihypertensive medication. Diabetes mellitus was defined as requirement for insulin or oral hypoglycemic drugs. Dyslipidemia was defined as a history of hypercholesterolemia or hypertriglyceridemia diagnosed and/or treated by a physician or the use of lipid-lowering agents. Smoking status was defined in three categories: current smoker — smoking exposure within the last 12 months; ex-smoker — life smoking exposure greater than 2 pack-years with no smoking in the last year; non-smoker — no smoking within the last 12 months and a life consumption of less than 2 pack-years. Family history of CAD was defined as having a first-degree relative with a history of myocardial infarction, coronary revascularization or sudden death at a young age (men <55 years; women <65 years). Metabolic syndrome was diagnosed according to the Adult Treatment Panel III (ATP III) criteria12.
Anthropometric measurementsAbdominal circumference was measured according to standard methods13, and body mass index (BMI) calculated from simultaneous measurements of height and weight.
Blood pressure and blood testsPrior to administration of MDCT, pre-medication, measurements of sitting heart rate and blood pressure were obtained and peripheral blood samples collected after overnight fasting.
MDCT acquisitionAll patients underwent an MDCT scan using a 64-slice CT scanner (Somatom Sensation 64, Siemens Medical Solutions, Forchheim, Germany), including three different acquisitions: one for abdominal fat quantification, the second for quantification of coronary artery calcification (CAC) and the third for coronary angiography plus epicardial adipose tissue quantification.
Abdominal fat assessmentTo assess abdominal fat, a single-slice abdominal CT scan was performed between L4 and L5, according to the method described by Borkan et al.14. The scan parameters were 120kV and 216 mAs with 5mm thickness. This resulted in an estimated radiation exposure of 0.06mSv. On the scan obtained, a cursor pointer was used to trace the abdominal visceral fat area, and the data were processed using a histogram-based statistical program according to the previously described method14. One expert, unaware of the patient's details, results for calcium score or CT angiography, measured abdominal fat distribution. The CT value of body fat ranged from -150 to -50 Hounsfield units (HU). Total abdominal fat area was measured and subcutaneous fat area was obtained by subtracting abdominal visceral fat from the total abdominal fat area. The ratio of visceral to subcutaneous fat (V/S ratio) was also calculated.
Agatston calcium score quantificationAll patients underwent a low-dose scan to assess CAC. The scan parameters for this acquisition were: collimation 24×1.2mm; gantry rotation time 330ms; pitch 0.2; tube voltage 120kV and tube current 190 mAs. Estimated radiation exposure was 2.1±0.4mSv. Image reconstruction of the calcium score acquisition was performed using an effective slice thickness of 3mm. CAC was reported as the mean Agatston score and was calculated using a detection threshold of 130 HU with semi-automated software (Syngo Calcium Scoring, Siemens Medical Solutions) as described previously15.
CAD assessmentFollowing the CAC acquisition, a CT angiography (CTA) acquisition was performed (collimation of 64×0.6mm; tube current 850 mAs; all other parameters similar to CAC acquisition scan). Tube current modulation with electrocardiographic pulsing was used to decrease the radiation dose, with full tube current applied at 60-65% of the RR interval. In patients with body weight <70kg, the tube voltage was reduced to 100kV. This resulted in an estimated radiation dose of 6.6±2.2mSv. Depending on the scan time, a bolus of 80 to 100ml of contrast (Ultravist®, iopromide 370mg/ml, Bayer Schering Pharma AG, Berlin, Germany) was injected at 5cc/min via a power injector (Stellant D®, Medrad Inc, Warrendale, PA, USA) followed by a 40-ml saline “chaser”, using a dedicated antecubital vein 18-gauge access catheter. A bolus-tracking technique was used, with a region of interest placed in the ascending aorta, set to detect a predefined threshold of 150 HU.
For assessment of CAD, multiphase sets of the reconstructed CTA images were processed on a dedicated workstation and analyzed for detection of at least one luminal diameter narrowing of >50% in any coronary artery segment16. Severely calcified segments (concentric vessel wall calcification precluding lumen assessment) were classified as positive for CAD.
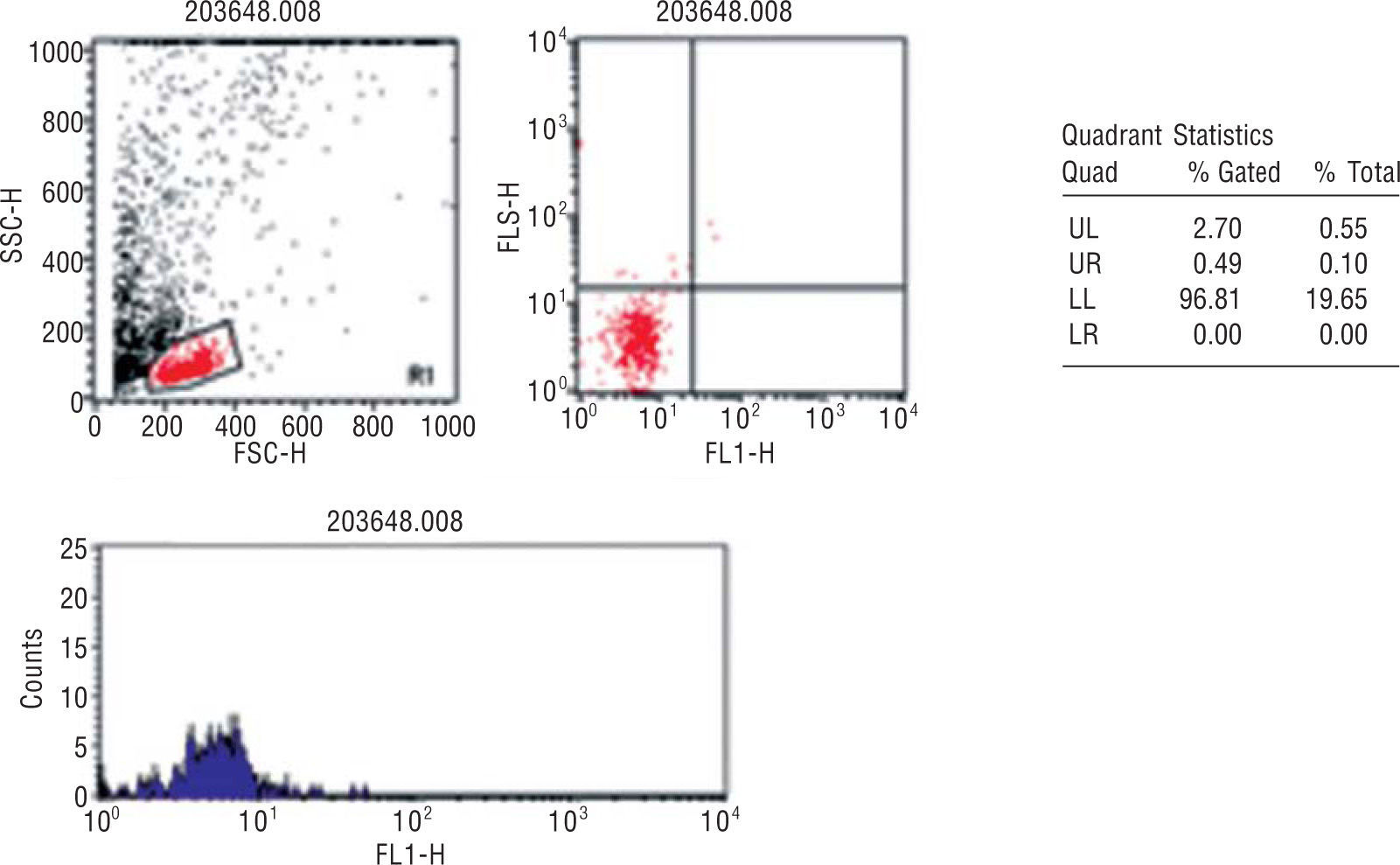
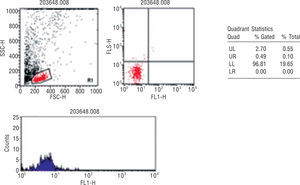
Quantification of circulating endothelial progenitor cellsBlood samples from all the participants were collected in tubes with EDTA and processed within 24h after extraction in a single reference center (Centro de Histocompatibilidade do Norte, Porto, Portugal). EPC quantification was performed according to the method described by Fadini et al. in 200517: 100ml of peripheral blood was incubated with monoclonal antibodies, FITC-conjugated anti-hCD34 (Pharmingen, Becton Dickinson) and PE-conjugated hKDR (R&D Systems). Unstained cells were used as controls. After incubation, the cells were washed with PBS, fixed with CellFix (Becton Dickinson) and subsequently counted in FACSCalibur (Becton Dickinson). The count was analyzed using fluorescence-1/fluorescence-2 dot-plot quadrant statistics and manual gating (Cell Quest Pro software, Becton Dickinson) (Figure 1).
Flow cytometry analysis of a representative blood sample. EPC detection was based on detection of CD34 and KDR surface markers in cell populations of low cytoplasmic granularity.
% Gated - % of CD34/KDR in region 1; % Total: total % of CD34/KDR counts; FL1: fluorescence 1 = CD34-FITC; FL2: fluorescence 2 = KDR-PE; FSC-H: forward scatter — height; SSC-H: side scatter — height.
Ejection fraction was calculated using dedicated software (Circulation®, Siemens Medical Solutions) for CT, based on the data acquired for CTA. For this purpose, multiphase reconstructions with 20 phases (5% increments) of the R-R cycle were obtained. Systolic and diastolic phases were suggested by the software and corrected by the operator. Semi-automatic detection of the endocardial border was used under operator control for end-systolic and end-diastolic volume quantification. For left ventricular mass quantification, the epicardial contour was manually traced on the end-diastolic phase.
Radiation exposureMean radiation exposure was estimated by the method proposed by the European Working Group for Guidelines on Quality Criteria in CT18.
The effective radiation doses for the CAC and CTA acquisitions were calculated by the product of the chest coefficient (0.014mSv/mGy per cm averaged between male and female models) and the dose-length product (DLP) obtained during each scan18. For the abdominal fat acquisition the abdominal coefficient (0.015mSv/mGy per cm averaged between male and female models)19 was used.
Statistical analysisContinuous variables were expressed as means and standard deviations. Distributions of continuous variables were examined using density plots and the Kolmogorov-Smirnov test. Associations between CD34+/KDR+ cells and potential predictors were examined with regression models. Models were defined as appropriate if the assumption was fulfilled for linear regression (normal distribution, heteroskedasticity and independence of residuals). Poisson regression was used for count data and non-parametric regression was used in other cases. The respective SAS codes used for linear regression were “proc glm”, for Poisson regression “proc genmod”, and for non-parametric tests “proc npar1way”. The type I error level was taken to be p=0.05. All calculations were carried out using the statistical software packages SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
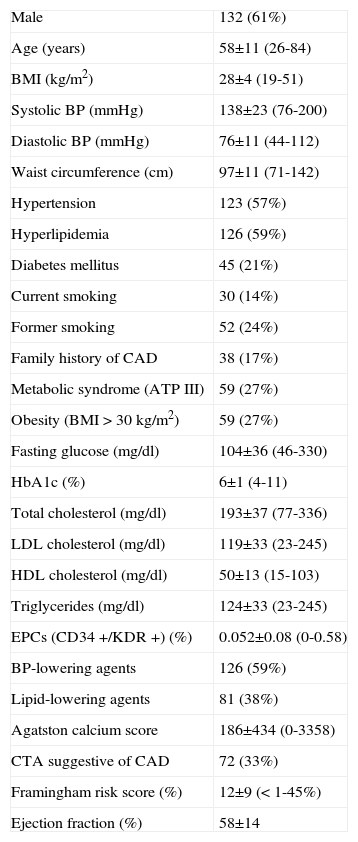
ResultsThe clinical characteristics and laboratory data of the 215 patients are summarized in Table 1. The final population consisted of 215 patients (132 male, 83 female) with a mean age of 58±11 years (min 26 to max 84).
Baseline characteristics of the study population (n=215)
| Male | 132 (61%) |
| Age (years) | 58±11 (26-84) |
| BMI (kg/m2) | 28±4 (19-51) |
| Systolic BP (mmHg) | 138±23 (76-200) |
| Diastolic BP (mmHg) | 76±11 (44-112) |
| Waist circumference (cm) | 97±11 (71-142) |
| Hypertension | 123 (57%) |
| Hyperlipidemia | 126 (59%) |
| Diabetes mellitus | 45 (21%) |
| Current smoking | 30 (14%) |
| Former smoking | 52 (24%) |
| Family history of CAD | 38 (17%) |
| Metabolic syndrome (ATP III) | 59 (27%) |
| Obesity (BMI >30kg/m2) | 59 (27%) |
| Fasting glucose (mg/dl) | 104±36 (46-330) |
| HbA1c (%) | 6±1 (4-11) |
| Total cholesterol (mg/dl) | 193±37 (77-336) |
| LDL cholesterol (mg/dl) | 119±33 (23-245) |
| HDL cholesterol (mg/dl) | 50±13 (15-103) |
| Triglycerides (mg/dl) | 124±33 (23-245) |
| EPCs (CD34+/KDR+) (%) | 0.052±0.08 (0-0.58) |
| BP-lowering agents | 126 (59%) |
| Lipid-lowering agents | 81 (38%) |
| Agatston calcium score | 186±434 (0-3358) |
| CTA suggestive of CAD | 72 (33%) |
| Framingham risk score (%) | 12±9 (<1-45%) |
| Ejection fraction (%) | 58±14 |
Values are means ± SD (min-max) unless otherwise indicated.
BMI: body mass index; BP: blood pressure; CAD: coronary artery disease; CTA: computed tomography angiography; EPCs: endothelial progenitor cells.
Based on the Framingham risk score20, the mean risk of developing CAD events during the ensuing 10 years for the entire population was 12±9%. As expected from a population referred for CT coronary angiography, without known CAD, this can be considered an overall low-to-intermediate risk population. Mean body mass index was 28±4kg/m2 (min 20 to max 49). Fifty-nine (27%) and 169 (79%) patients were obese or overweight (BMI >30 or 25kg/m2, respectively). Mean abdominal circumference was 97±11cm. Mean visceral abdominal fat was 157±75 (range 21-488), 168±39 in men, 139±68 in women. Seventy-two patients (33%) had a positive MDCT indicating at least one cardiac vessel with a ≥50% stenosis.
Flow cytometry was used to determine the number of circulating peripheral blood CD34+/KDR+ cells (EPCs). The mean EPC count in peripheral blood, expressed as a percentage of total white blood cell counts, was 0.052±0.08% (min 0.0 to max 0.58%; 0.047±0.007% in men; 0.058±0.01% in women), and the median was 0.02% (interquartile range 0.0 to 0.075%).
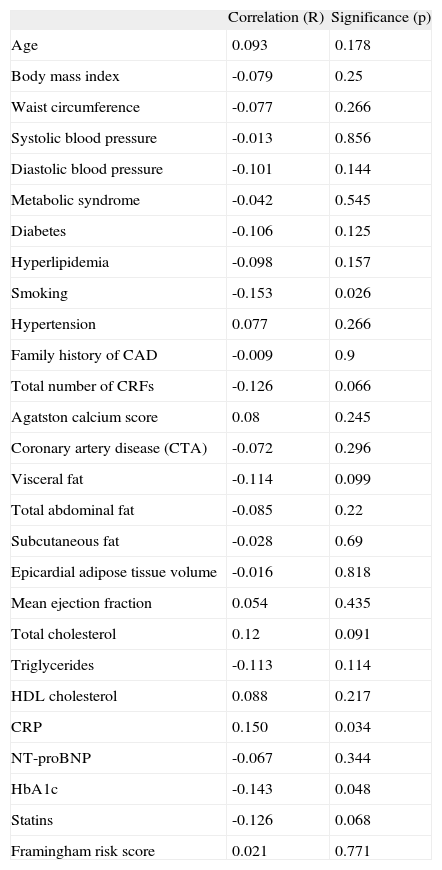
The relations of circulating EPC counts in peripheral blood to pre-specified parameters and risk factors are presented in Table 2.
Bivariate correlation between EPC counts in peripheral blood and pre-specified baseline parameters and risk factors (n=212)
| Correlation (R) | Significance (p) | |
| Age | 0.093 | 0.178 |
| Body mass index | -0.079 | 0.25 |
| Waist circumference | -0.077 | 0.266 |
| Systolic blood pressure | -0.013 | 0.856 |
| Diastolic blood pressure | -0.101 | 0.144 |
| Metabolic syndrome | -0.042 | 0.545 |
| Diabetes | -0.106 | 0.125 |
| Hyperlipidemia | -0.098 | 0.157 |
| Smoking | -0.153 | 0.026 |
| Hypertension | 0.077 | 0.266 |
| Family history of CAD | -0.009 | 0.9 |
| Total number of CRFs | -0.126 | 0.066 |
| Agatston calcium score | 0.08 | 0.245 |
| Coronary artery disease (CTA) | -0.072 | 0.296 |
| Visceral fat | -0.114 | 0.099 |
| Total abdominal fat | -0.085 | 0.22 |
| Subcutaneous fat | -0.028 | 0.69 |
| Epicardial adipose tissue volume | -0.016 | 0.818 |
| Mean ejection fraction | 0.054 | 0.435 |
| Total cholesterol | 0.12 | 0.091 |
| Triglycerides | -0.113 | 0.114 |
| HDL cholesterol | 0.088 | 0.217 |
| CRP | 0.150 | 0.034 |
| NT-proBNP | -0.067 | 0.344 |
| HbA1c | -0.143 | 0.048 |
| Statins | -0.126 | 0.068 |
| Framingham risk score | 0.021 | 0.771 |
CAD: coronary artery disease; CRFs: cardiovascular risk factors; CRP: C-reactive protein; CTA: computed tomography angiography; NT-proBNP: N-terminal pro b-type natriuretic peptide.
Surprisingly age (p=0.247) and gender (p=0.067) were not significantly associated with EPC counts in peripheral blood.
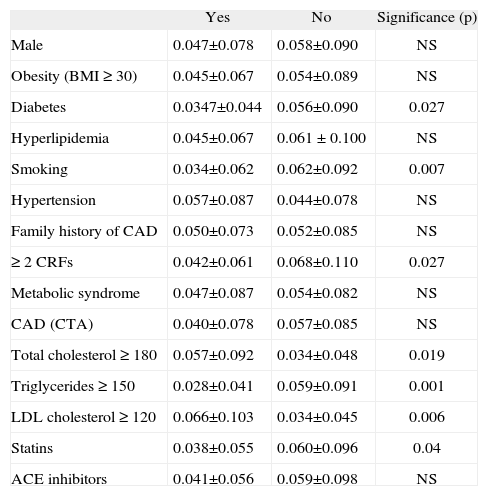
EPC levels in peripheral blood tended to be inversely related to the total number of cardiovascular risk factors (p=0.066). Only diabetes mellitus (mean difference -0.021; p=0.027) and smoking (mean difference -0.029; p=0.007) were associated with lower levels of EPCs. Lower EPC counts were also found in patients with hypertriglyceridemia (triglycerides >150mg/dl), p=0.023.
A weak but significant inverse correlation was found between HbA1c and EPC counts (p=0.048). By contrast, CRP was directly correlated with EPC counts (p=0.027).
EPC levels were not significantly associated with the presence of significant stenoses as defined by MDCT coronary angiography or with the global atherosclerotic burden, as defined by the Agatston calcium score.
Circulating EPC levels were not significantly related to measurements of obesity, visceral fat, or epicardial adipose tissue, or to the presence of metabolic syndrome. However, in diabetic patients visceral fat was inversely related to EPC counts (p=0.037) (Table 3).
Mean circulating EPC counts in peripheral blood in different subpopulations
| Yes | No | Significance (p) | |
| Male | 0.047±0.078 | 0.058±0.090 | NS |
| Obesity (BMI ≥30) | 0.045±0.067 | 0.054±0.089 | NS |
| Diabetes | 0.0347±0.044 | 0.056±0.090 | 0.027 |
| Hyperlipidemia | 0.045±0.067 | 0.061 ±0.100 | NS |
| Smoking | 0.034±0.062 | 0.062±0.092 | 0.007 |
| Hypertension | 0.057±0.087 | 0.044±0.078 | NS |
| Family history of CAD | 0.050±0.073 | 0.052±0.085 | NS |
| ≥2 CRFs | 0.042±0.061 | 0.068±0.110 | 0.027 |
| Metabolic syndrome | 0.047±0.087 | 0.054±0.082 | NS |
| CAD (CTA) | 0.040±0.078 | 0.057±0.085 | NS |
| Total cholesterol ≥180 | 0.057±0.092 | 0.034±0.048 | 0.019 |
| Triglycerides ≥150 | 0.028±0.041 | 0.059±0.091 | 0.001 |
| LDL cholesterol ≥120 | 0.066±0.103 | 0.034±0.045 | 0.006 |
| Statins | 0.038±0.055 | 0.060±0.096 | 0.04 |
| ACE inhibitors | 0.041±0.056 | 0.059±0.098 | NS |
BMI: body mass index; CAD: coronary artery disease; CRFs: cardiovascular risk factors; CTA: computed tomography angiography.
According to our data, in patients without known CAD referred for MDCT, circulating EPC counts in peripheral blood cannot be significantly predicted from baseline population characteristics, anthropometric measures, adiposity measurements, clinical context, risk factors or even CTA. Some correlations were found between cardiovascular risk factors and circulating EPC levels; however, those relations do not appear to be sufficiently strong to be used for predicting EPC levels in a clinical context.
Certain factors have been suggested as influencing peripheral blood EPC concentrations3,21. Hill et al. found an inverse relation between the number of circulating EPCs and the Framingham cardiovascular risk score (FRS) in 45 patients without known CAD21. However, a later study based on a larger population (571 patients) showed a positive relation between the FRS and EPCs22. In our low-to-intermediate risk population no significant correlation between the FRS and EPCs was found. Concerning cardiovascular risk factors, smoking appears to be the best predictor for lower EPC levels; in our study, it was the only risk factor significantly associated with lower EPC counts. Diabetes (both type 1 and type 2) has also been related to lower EPC concentrations in peripheral blood45. Our study confirms this finding, as EPC levels were significantly lower in diabetic patients and inversely related to glycated hemoglobin. By contrast, unexpectedly23, hypertension was not significantly associated with EPC levels in peripheral blood. Interestingly, LDL cholesterol was found to correlate positively with circulating EPC levels, while patients with higher triglyceride levels tended to have lower levels. This is in disagreement with previous studies3,21,24. Statins have been reported as increasing the number and mobilization of circulating EPCs, by stimulating the differentiation and maturation of bone marrow precursors into EPCs25-28. However, a recent study in patients with documented CAD showed an inverse correlation between statin dose in continuous treatment and EPC concentration in peripheral blood8. In our population, patients under chronic therapy with statins were found to have lower levels of circulating EPCs (0.038±0.055 vs. 0.060±0.096, p=0.04). Interpretation of these findings is difficult, since there is significant collinearity and interaction between the parameters. For instance, patients on statins are more likely to have a history of dyslipidemia and hence greater exposure to vascular damage.
Both myocardial and peripheral ischemia have been associated with a rapid increase in circulating EPCs. The same has been reported for vascular injuries, including bypass surgery29-31. Systemic inflammation, expressed by an increase in CRP, also correlated positively with the number of circulating EPCs32. Our population excluded patients with known CAD and did not include patients with acute coronary syndromes or post-coronary artery bypass grafting. In this stable population, we were able to confirm the positive correlation of circulating EPCs with CRP expression.
Is it possible to predict which patients will respond to EPC-promoting therapies?EPCs appear to be the cornerstone of endogenous vascular regeneration after ischemic injury. Monitoring circulating EPCs (CD34+/KDR+) could therefore be useful for identifying patients at risk. This concept is driving research in this field as therapies aimed at stimulating endogenous vascular regeneration and repair become a reality10,11. Stents that “capture” EPCs onto their surface have been recently developed using bioengineering techniques. This approach aims to accelerate vascular regeneration after coronary angioplasty, promoting rapid re-endothelialization. This may have the advantage of reducing restenosis and intrastent thrombosis33-35. However, the application of therapies based on mobilization of endogenous EPCs requires a deeper knowledge of possible inter-individual variations in circulating EPC levels. It is thus important to recognize the normal distribution patterns of these cells in different populations and to identify factors that may influence their concentration and mobilization.
Our study aimed to investigate whether EPC counts could be predicted with any degree of certainty on the basis of patients' baseline characteristics. The results show that, at least in this population of patients with low-to-intermediate CV risk without known CAD, only very limited prediction is possible: all the previously described associations (pre-specified in our study) were found to be weak or even non-significant.
Is there any relation between circulating EPC levels and CAD or coronary atherosclerotic burden? And with visceral adiposity? Could EPC levels be used as independent markers of cardiovascular risk?Studies on the impact of obesity on EPC concentrations in peripheral blood are contradictory8,36,37. In our study no correlation was detected between EPCs and BMI and no significant differences were found between obese and non-obese patients. Similarly, no significant relation was found between EPC levels in peripheral blood and abdominal circumference, abdominal fat or epicardial fat.
Some authors have proposed that, as circulating EPC levels may fall in the preclinical stage of the atherosclerotic process, quantification of EPCs in peripheral blood could provide useful additional information to classical risk factors for global cardiovascular risk assessment9,38,39. In our study no significant correlation was shown between EPC counts and calcium score (as a marker of atherosclerotic burden). Similarly, and in contrast to a smaller study by Wang et al.6, we found no correlation between the presence or severity of CAD and the number of circulating EPCs. This possible relation is still the subject of debate, as contradictory data have been published40,41.
Only patients without known CAD referred for MDCT were included in this single-center exploratory analysis, and so this sample may not represent the general population. The potential value of quantifying circulating EPCs for cardiovascular risk prediction was not tested, since there was no follow-up of events. Furthermore, only the number of circulating EPCs in peripheral blood — and not EPC activity or mobility — was analyzed in our study; this should be taken in consideration when comparing our results with studies in which these were measured.
Finally, the lack of a standardized, verified, and universally accepted EPC phenotype is an important limitation that is common to all studies focusing on vasculogenesis42. To minimize this, the cell population (CD34+/KDR+) chosen and the quantification method used in our study are among the most widely used in publications concerning EPC characterization.
ConclusionAlthough some correlations were found between circulating EPC levels and cardiovascular risk factors, namely diabetes and smoking (inverse relation) and CRP (direct relation), those relations were found to be too weak for EPC prediction in a clinical context. Therefore, we conclude that in patients without known CAD referred for MDCT, EPC levels in peripheral blood cannot be significantly predicted from knowledge of patient anamnesis, risk factors, visceral fat, CAC or CTA.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Siemens Medical Solutions and Terarecon Inc. for support with applications. Thanks are extended to all the nurses involved in patient preparation, for their dedication and hard work.