The Portuguese Registry of Acute Coronary Syndromes (ProACS) has completed 15 years of continuous and prospective activity. We present an overall picture of the data from this powerful tool. Up to 2016, 45141 records were included, mostly male (71%), and with a mean age of 66 years. Baseline characteristics remained stable over the years. Of the overall population, 44% of cases were ST-elevation myocardial infarction (STEMI). Over the years there was a significant improvement in compliance with international guidelines, in terms of both diagnostic and therapeutic procedures, as well as for medication. In particular, the rate of reperfusion in STEMI increased to 84%, mainly by primary percutaneous coronary intervention (only 5.2% were treated with thrombolysis in 2016). By contrast, timings in STEMI did not change significantly. Improvements in treatment were accompanied by a reduction in in-hospital mortality from 6.7% in 2002 to 2.5% in 2016 in the overall population. This registry enables analysis of the management and results of acute coronary syndromes over time in Portugal, and hence assessment of improvements in quality of care.
O Registo Nacional de Síndromes Coronárias Agudas (SCA) completa agora 15 anos de atividade contínua prospetiva. Descrevemos no presente trabalho os dados gerais obtidos a partir dessa poderosa ferramenta. Incluíram-se até ao momento 45.141 registos, a maioria do sexo masculino (71%), com idade média de 66 anos, com características basais que se têm mantido estáveis. Da população total, 44% são enfartes com elevação do segmento ST (EMCST). Ao longo dos anos verificou-se uma melhoria muito significativa no cumprimento das recomendações internacionais, quer no que diz respeito aos procedimentos de diagnóstico e terapêutica quer na medicação efetuada. Em particular, no que diz respeito ao EMCST, as taxas de reperfusão aumentaram progressivamente, são hoje de 84% e preferencialmente com recurso a angioplastia primária (fibrinólise atualmente em apenas 5,2% dos casos). Pelo contrário, os intervalos temporais no enfarte não se têm modificado significativamente. As melhorias no tratamento têm-se acompanhado por melhoria sustentada da mortalidade intra-hospitalar, que era 6,7% em 2002 e hoje 2,5% na população global. Este registo tem permitido uma análise temporal da abordagem e resultados do tratamento das SCA em Portugal e permite aferir a qualidade dos cuidados.
Despite a progressive decline in recent years, diseases of the circulatory system are still the leading cause of death in Portugal (29.5% in 2013).1 Of these diseases, stroke is the most common cause of death, while acute coronary syndromes (ACS) caused 37% of deaths in 2013, with a rate of 22.2/100000 individuals.1 In Europe as a whole, coronary artery disease accounts for about 20% of all deaths.2 The observed reduction in mortality is directly related to improvements in medical care, especially through the introduction of new drugs, as well as a more interventional approach. However, clinical practice continues to vary significantly.
International guidelines issued by various medical societies recommend the establishment of registries in order to evaluate whether the recommended measures are in accordance with current evidence.3 Better compliance with the guidelines is associated with reductions in adverse events, although in clinical practice, measures recommended in the guidelines are usually implemented later than recommended.4 Clinical trials designed to determine whether a specific diagnostic or therapeutic approach is beneficial are carried out in highly controlled populations selected according to strict inclusion and exclusion criteria. The consequence is that the results of such trials cannot always be transferred to real patients in highly variable environments who do not fulfill these selection criteria. Registries are closer to the reality of patient populations and enable a more accurate assessment of therapeutic strategies.
The first Portuguese cardiology registries were established in the 1990s, under the aegis of the Portuguese Society of Cardiology (SPC), a pioneer in this field, particularly with its short-term 1999 national registry on in-hospital management of myocardial infarction (MI).5 In 2002, recognizing the need to improve knowledge of how ACS were managed nationwide, the Board of the SPC established the National Center for Data Collection in Cardiology (CNCDC) to centralize the relevant data and to support its analysis. This resulted in the simultaneous creation of the CNCDC's first registries, the Portuguese Registry of Acute Coronary Syndromes (ProACS) and the National Registry of Interventional Cardiology (RNCI). ProACS, in which several cardiology departments in Portuguese hospitals participate, aims to characterize patients and diagnostic and therapeutic approaches to ACS in Portugal, to monitor compliance with clinical guidelines and recommended timings, and to assess the impact of implementing specific recommendations.6 The guidelines themselves have also undergone changes over the years, and ProACS allows their impact to be analyzed at a national level.
In 2009, data from the registry's first seven years were published. These showed that despite marked improvements in some parameters, application of the guidelines for ACS treatment in clinical practice remained suboptimal, similarly to what had been seen in 20026,7 and to data from registries in other countries. In 2010, it was considered necessary to include more variables in the data collection, particularly on percutaneous coronary intervention (PCI), and the data collection sheet was accordingly reformulated, although the previous operating model remained unchanged. This marked the second phase of the ProACS registry.
The present document summarizes the information collected over the 15 years of continuous activity of the ProACS registry.
MethodsThe ProACS is a continuous prospective observational registry, under the aegis of the SPC and coordinated by the CNCDC. All cardiology departments at Portuguese hospitals were invited to participate. Inclusion of patients began on January 1, 2002 and has continued uninterrupted to the present. The inclusion and exclusion criteria, as well as the data collection sheet, have been described in previous publications.6,7 Briefly, each center is asked to consecutively include patients hospitalized with a diagnosis of ACS (ST-elevation myocardial infarction [STEMI], non-ST-elevation myocardial infarction [NSTEMI] or unstable angina), based on clinical status, electrocardiogram (ECG) and myocardial necrosis biomarkers. The data collected include demographics, baseline characteristics, changes in laboratory test results, clinical course, treatment, information on PCI, and data at discharge and at six-month (in the first phase of the registry) or one-year (in the second phase) follow-up. Data were initially presented on paper and then transferred to a digital database, but since 2004 have been submitted directly in electronic form.
The data are held centrally at the CNCDC in Coimbra, in an anonymized database of all included patients. The data entered are validated in two stages: firstly by the computer system using auditing software at data entry, which identifies missing or inadmissible values, and secondly by the investigator who enters the data into the system. The registry is approved by the Portuguese Data Protection Authority (no. 3140/2010), is registered at ClinicalTrials.gov (NCT 01642329), and is supervised by an Executive Committee appointed for the purpose.
Statistical analysisThis analysis included all patients correctly entered in the registry and validated between January 1, 2002 and December 31, 2016. Continuous variables with normal distribution are presented as means and standard deviation or as medians and interquartile range otherwise. Categorical variables are presented as percentages and were compared using the chi-square test. Changes over time in therapeutic interventions, baseline characteristics and prognosis (mortality) were assessed.
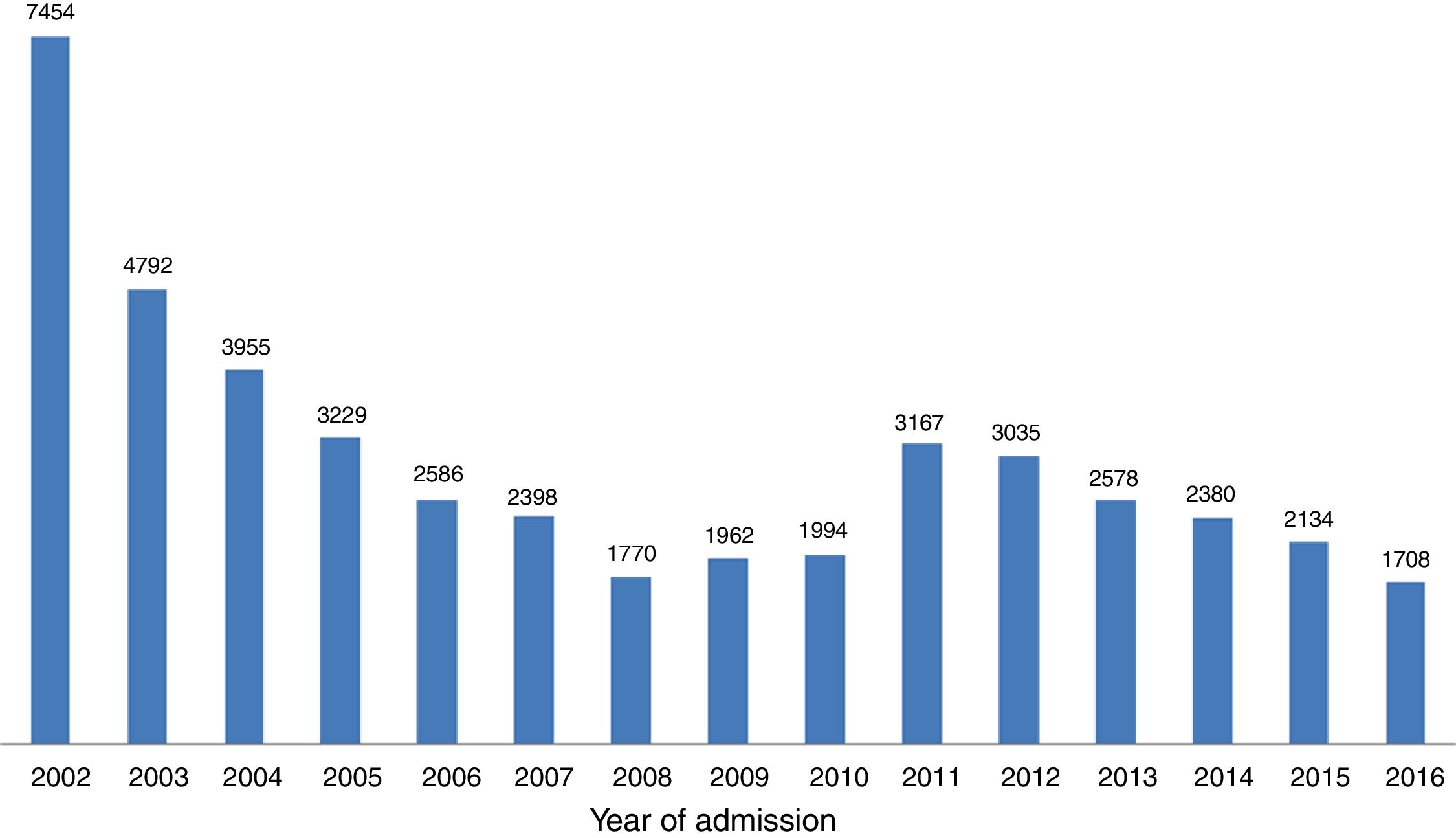
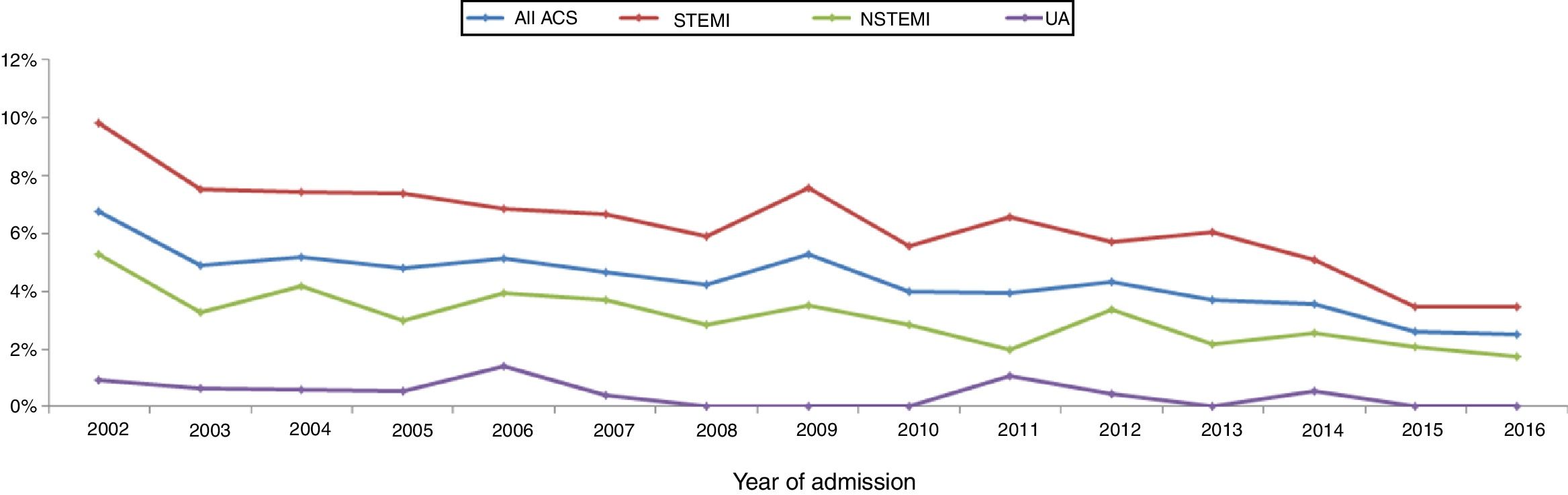
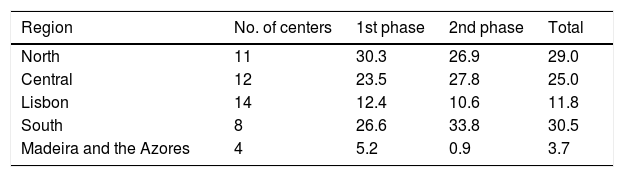
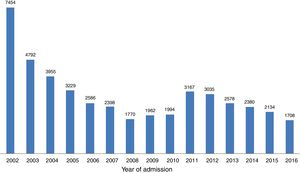
ResultsOver the 15-year study period, 49 centers participated, including 11 university hospitals, 12 hospitals with cardiac surgery and 25 hospitals with catheterization laboratories. Eleven of these centers are in the North region, 12 in the Central region, 22 in the South and Lisbon regions and four in the Autonomous Regions of Madeira and the Azores (Table 1). In the first phase, 29244 records were included, and 15897 were recorded in the second, making a total of 45141 records. With regard to the volume of patients included, 25 centers included fewer than 500 patients, nine included between 500 and 1000, and 15 centers included more than 1000 patients. The number of actively participating centers decreased over time, with 46 in the first phase and 25 in the second. The mean number of records included per center was 636 in both phases. The mean number of patients per year in the first phase (3342 records/year) fell in the second (2543 records/year), although many fluctuations were found in annual trends (Figure 1).
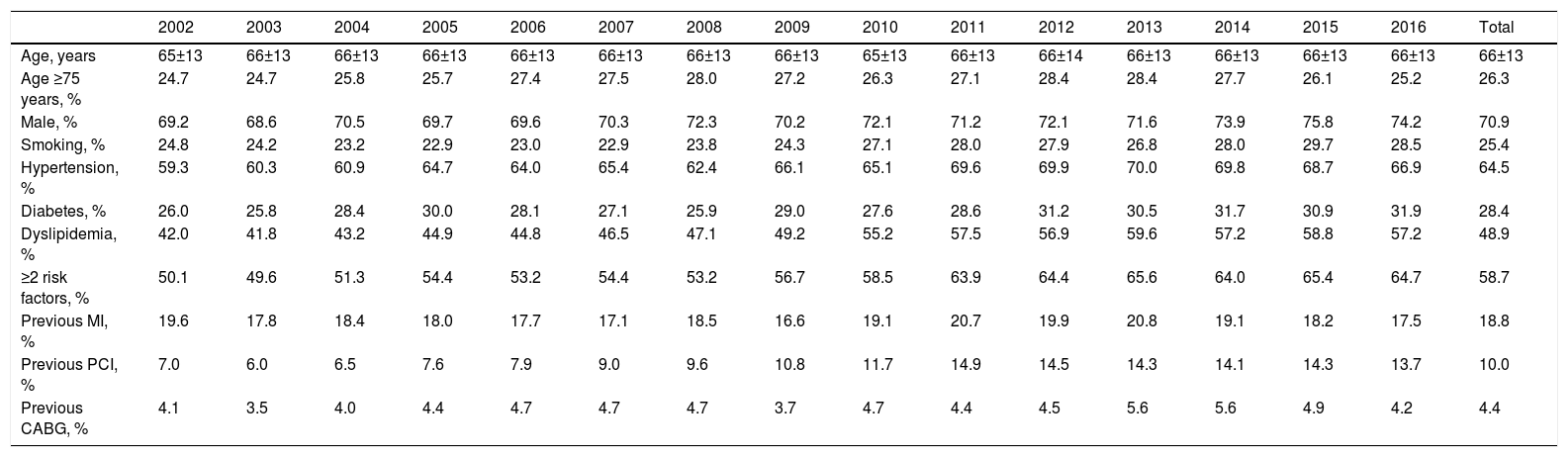
Among the 45141 records included, the mean age was 66±13 years and 70.9% were male (26.0% aged ≥75 years); demographic characteristics remained stable over the years. Baseline demographic characteristics, cardiovascular risk factors and previous cardiovascular history are shown in Table 2. The most prevalent risk factor was hypertension (64.5%), followed by dyslipidemia (48.9%), diabetes (28.4%) and smoking (25.4%). The prevalence of cardiovascular risk factors and of a history of previous PCI has increased over time. In terms of comorbidities, 7.2% of patients had a history of stroke or transient ischemic attack, 4.0% had known peripheral vascular disease, 5.9% renal failure, 4.8% history of cancer and 5.5% chronic obstructive pulmonary disease. With the exception of peripheral vascular disease, which increased from 3.2% to 5.7%, these rates remained stable over the study period.
Baseline characteristics of patients included in the ProACS registry.
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 65±13 | 66±13 | 66±13 | 66±13 | 66±13 | 66±13 | 66±13 | 66±13 | 65±13 | 66±13 | 66±14 | 66±13 | 66±13 | 66±13 | 66±13 | 66±13 |
| Age ≥75 years, % | 24.7 | 24.7 | 25.8 | 25.7 | 27.4 | 27.5 | 28.0 | 27.2 | 26.3 | 27.1 | 28.4 | 28.4 | 27.7 | 26.1 | 25.2 | 26.3 |
| Male, % | 69.2 | 68.6 | 70.5 | 69.7 | 69.6 | 70.3 | 72.3 | 70.2 | 72.1 | 71.2 | 72.1 | 71.6 | 73.9 | 75.8 | 74.2 | 70.9 |
| Smoking, % | 24.8 | 24.2 | 23.2 | 22.9 | 23.0 | 22.9 | 23.8 | 24.3 | 27.1 | 28.0 | 27.9 | 26.8 | 28.0 | 29.7 | 28.5 | 25.4 |
| Hypertension, % | 59.3 | 60.3 | 60.9 | 64.7 | 64.0 | 65.4 | 62.4 | 66.1 | 65.1 | 69.6 | 69.9 | 70.0 | 69.8 | 68.7 | 66.9 | 64.5 |
| Diabetes, % | 26.0 | 25.8 | 28.4 | 30.0 | 28.1 | 27.1 | 25.9 | 29.0 | 27.6 | 28.6 | 31.2 | 30.5 | 31.7 | 30.9 | 31.9 | 28.4 |
| Dyslipidemia, % | 42.0 | 41.8 | 43.2 | 44.9 | 44.8 | 46.5 | 47.1 | 49.2 | 55.2 | 57.5 | 56.9 | 59.6 | 57.2 | 58.8 | 57.2 | 48.9 |
| ≥2 risk factors, % | 50.1 | 49.6 | 51.3 | 54.4 | 53.2 | 54.4 | 53.2 | 56.7 | 58.5 | 63.9 | 64.4 | 65.6 | 64.0 | 65.4 | 64.7 | 58.7 |
| Previous MI, % | 19.6 | 17.8 | 18.4 | 18.0 | 17.7 | 17.1 | 18.5 | 16.6 | 19.1 | 20.7 | 19.9 | 20.8 | 19.1 | 18.2 | 17.5 | 18.8 |
| Previous PCI, % | 7.0 | 6.0 | 6.5 | 7.6 | 7.9 | 9.0 | 9.6 | 10.8 | 11.7 | 14.9 | 14.5 | 14.3 | 14.1 | 14.3 | 13.7 | 10.0 |
| Previous CABG, % | 4.1 | 3.5 | 4.0 | 4.4 | 4.7 | 4.7 | 4.7 | 3.7 | 4.7 | 4.4 | 4.5 | 5.6 | 5.6 | 4.9 | 4.2 | 4.4 |
CABG: coronary artery bypass grafting; MI: myocardial infarction; PCI: percutaneous coronary intervention.
STEMI accounted for 43.8% of all records, a proportion that remained stable over time. Overall, clinical signs of heart failure were seen in 17.9% (Killip class ≥2) and 2.0% were in cardiogenic shock. The proportions of patients in Killip class ≥2 and in cardiogenic shock fell between 2002 and 2016, from 22.2% to 12.5% (p<0.001) and from 2.2% to 1.2% (p=0.011), respectively. The most frequent location for STEMI on the ECG was anterior (45-50%). Cardiac rhythm at admission was only recorded from 2010, since when the percentage of patients in atrial fibrillation (AF) fell from 8.1% to 6.3% (p<0.001).
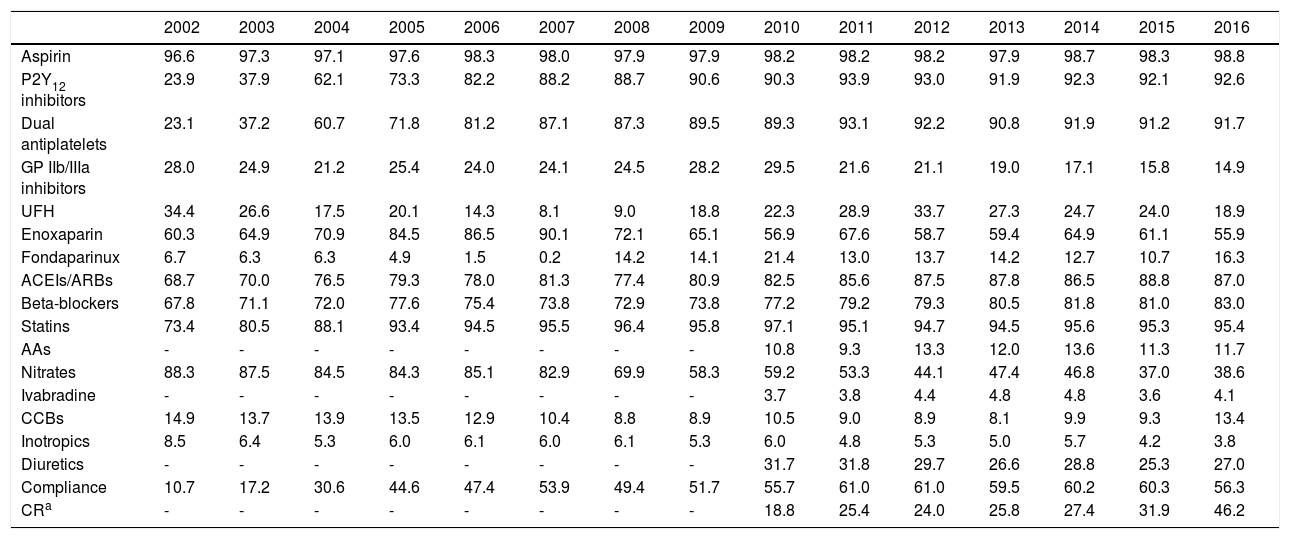
In-hospital treatmentWith regard to treatment during hospital stay (Table 3), the use of aspirin remained stable, while there was a progressive increase in the use of P2Y12 inhibitors (23.9% to 92.6%, p<0.001) and of dual antiplatelet therapy (23.1% to 91.7%, p<0.001). The use of glycoprotein (GP) IIb/IIIa inhibitors decreased, although it remained stable for the last two years (around 15%). With respect to other antithrombotic therapy, unfractionated heparin tended to be replaced by enoxaparin until 2007, although this trend then reversed; low molecular weight heparin remains the first-line treatment. Use of fondaparinux was initially very low, reaching its lowest point in 2007, but increased progressively thereafter, reaching about 15%.
Treatment during hospitalization (% of patients).
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin | 96.6 | 97.3 | 97.1 | 97.6 | 98.3 | 98.0 | 97.9 | 97.9 | 98.2 | 98.2 | 98.2 | 97.9 | 98.7 | 98.3 | 98.8 |
| P2Y12 inhibitors | 23.9 | 37.9 | 62.1 | 73.3 | 82.2 | 88.2 | 88.7 | 90.6 | 90.3 | 93.9 | 93.0 | 91.9 | 92.3 | 92.1 | 92.6 |
| Dual antiplatelets | 23.1 | 37.2 | 60.7 | 71.8 | 81.2 | 87.1 | 87.3 | 89.5 | 89.3 | 93.1 | 92.2 | 90.8 | 91.9 | 91.2 | 91.7 |
| GP IIb/IIIa inhibitors | 28.0 | 24.9 | 21.2 | 25.4 | 24.0 | 24.1 | 24.5 | 28.2 | 29.5 | 21.6 | 21.1 | 19.0 | 17.1 | 15.8 | 14.9 |
| UFH | 34.4 | 26.6 | 17.5 | 20.1 | 14.3 | 8.1 | 9.0 | 18.8 | 22.3 | 28.9 | 33.7 | 27.3 | 24.7 | 24.0 | 18.9 |
| Enoxaparin | 60.3 | 64.9 | 70.9 | 84.5 | 86.5 | 90.1 | 72.1 | 65.1 | 56.9 | 67.6 | 58.7 | 59.4 | 64.9 | 61.1 | 55.9 |
| Fondaparinux | 6.7 | 6.3 | 6.3 | 4.9 | 1.5 | 0.2 | 14.2 | 14.1 | 21.4 | 13.0 | 13.7 | 14.2 | 12.7 | 10.7 | 16.3 |
| ACEIs/ARBs | 68.7 | 70.0 | 76.5 | 79.3 | 78.0 | 81.3 | 77.4 | 80.9 | 82.5 | 85.6 | 87.5 | 87.8 | 86.5 | 88.8 | 87.0 |
| Beta-blockers | 67.8 | 71.1 | 72.0 | 77.6 | 75.4 | 73.8 | 72.9 | 73.8 | 77.2 | 79.2 | 79.3 | 80.5 | 81.8 | 81.0 | 83.0 |
| Statins | 73.4 | 80.5 | 88.1 | 93.4 | 94.5 | 95.5 | 96.4 | 95.8 | 97.1 | 95.1 | 94.7 | 94.5 | 95.6 | 95.3 | 95.4 |
| AAs | - | - | - | - | - | - | - | - | 10.8 | 9.3 | 13.3 | 12.0 | 13.6 | 11.3 | 11.7 |
| Nitrates | 88.3 | 87.5 | 84.5 | 84.3 | 85.1 | 82.9 | 69.9 | 58.3 | 59.2 | 53.3 | 44.1 | 47.4 | 46.8 | 37.0 | 38.6 |
| Ivabradine | - | - | - | - | - | - | - | - | 3.7 | 3.8 | 4.4 | 4.8 | 4.8 | 3.6 | 4.1 |
| CCBs | 14.9 | 13.7 | 13.9 | 13.5 | 12.9 | 10.4 | 8.8 | 8.9 | 10.5 | 9.0 | 8.9 | 8.1 | 9.9 | 9.3 | 13.4 |
| Inotropics | 8.5 | 6.4 | 5.3 | 6.0 | 6.1 | 6.0 | 6.1 | 5.3 | 6.0 | 4.8 | 5.3 | 5.0 | 5.7 | 4.2 | 3.8 |
| Diuretics | - | - | - | - | - | - | - | - | 31.7 | 31.8 | 29.7 | 26.6 | 28.8 | 25.3 | 27.0 |
| Compliance | 10.7 | 17.2 | 30.6 | 44.6 | 47.4 | 53.9 | 49.4 | 51.7 | 55.7 | 61.0 | 61.0 | 59.5 | 60.2 | 60.3 | 56.3 |
| CRa | - | - | - | - | - | - | - | - | 18.8 | 25.4 | 24.0 | 25.8 | 27.4 | 31.9 | 46.2 |
Regarding other disease-modifying drugs, use of renin-angiotensin system blockers, beta-blockers and statins increased over the years, with 95% of patients receiving statins and around 85% receiving the other two drug classes by the end of the study period. This corresponds to increases of 26%, 22% and 30%, respectively, compared to 2002 (p<0.001 for all comparisons). Use of nitrates fell to less than 40% of patients, and significant falls were also seen in the use of inotropics and diuretics. Use of aldosterone antagonists and ivabradine, only recorded since 2010, remained stable, at around 12% and 4%, respectively.
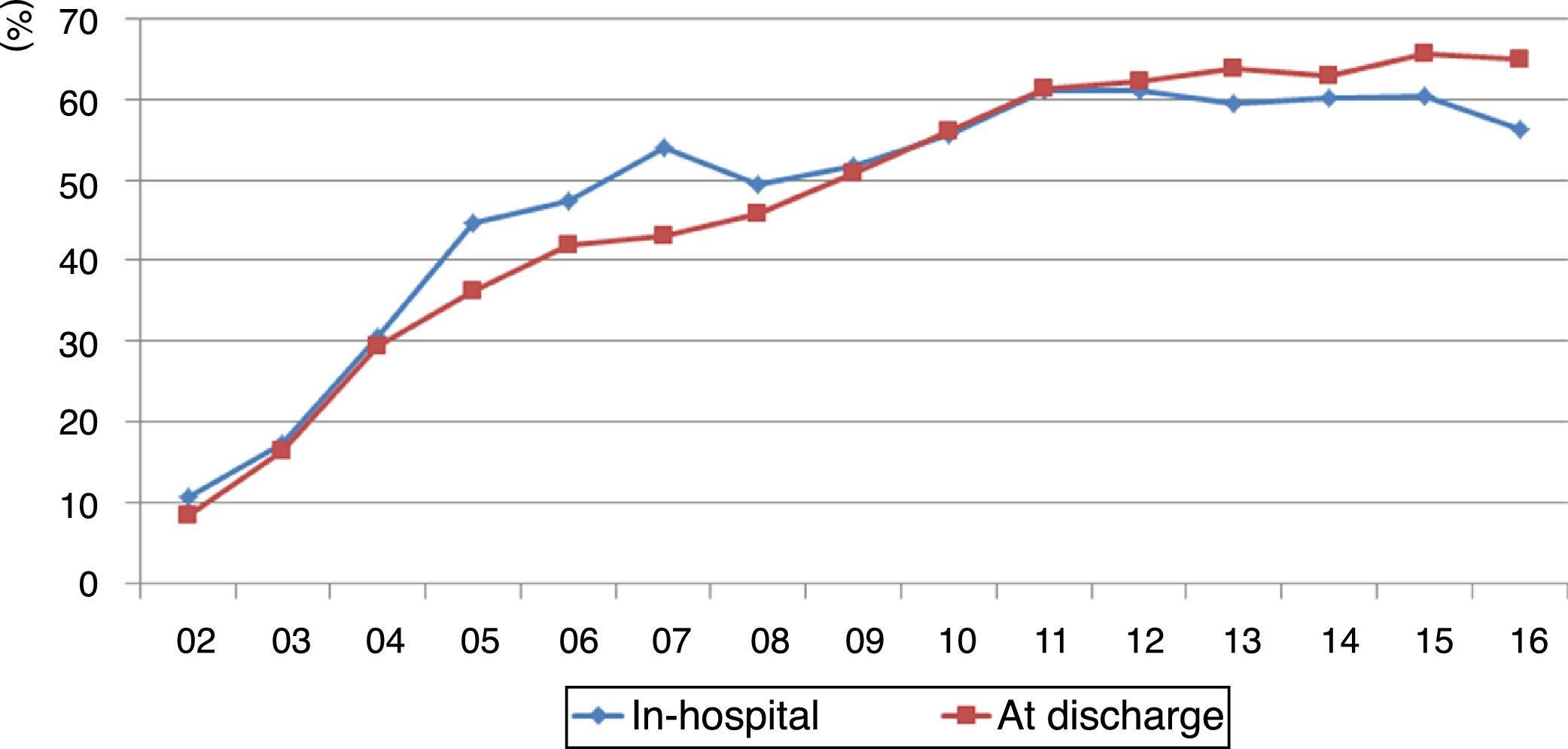
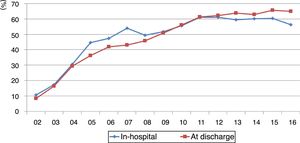
Compliance with the guidelines for dual antiplatelet therapy, parenteral anticoagulation, renin-angiotensin system blockers, beta-blockers and statins improved significantly, at around 60% since 2011, albeit with a slight fall in 2016 from 60.3% to 56.3% (p=0.014) (Figure 2).
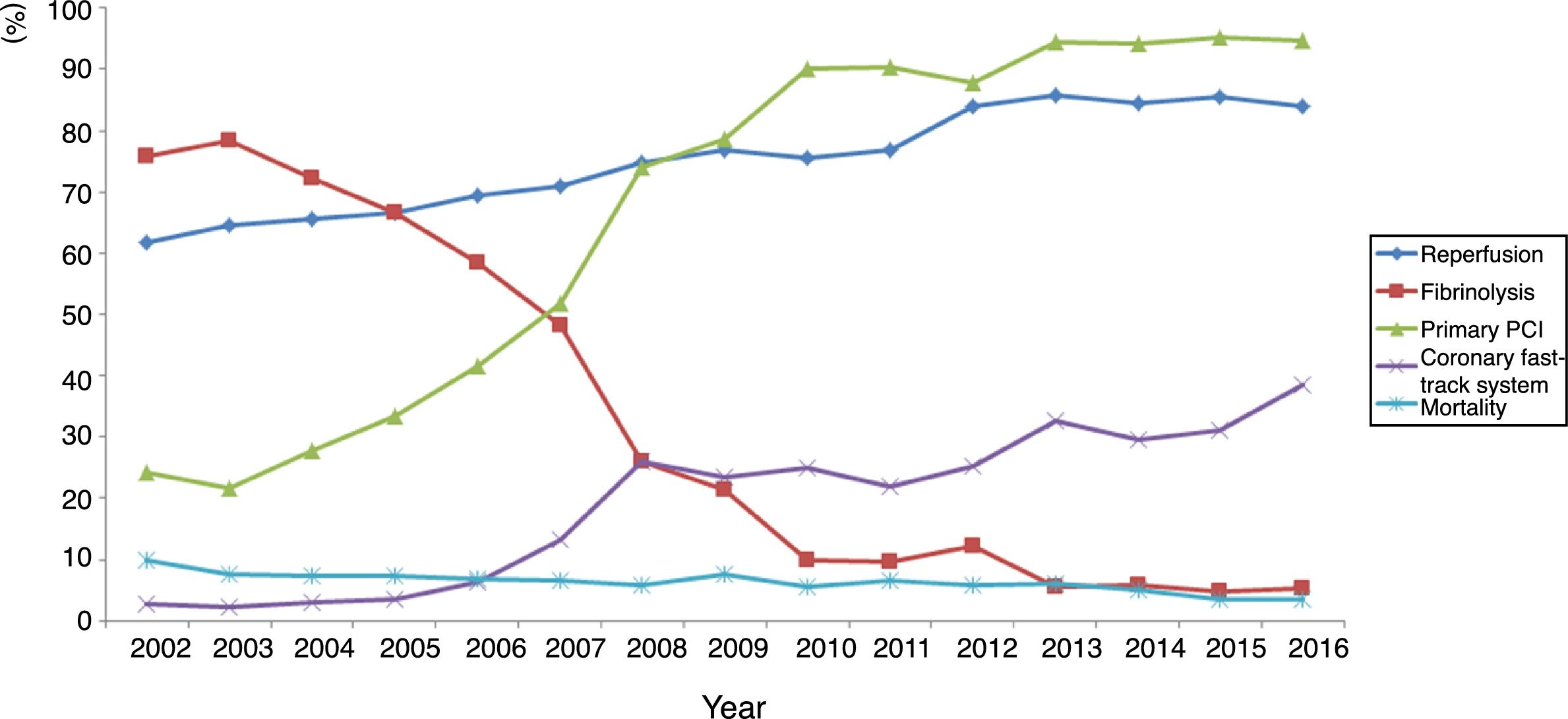
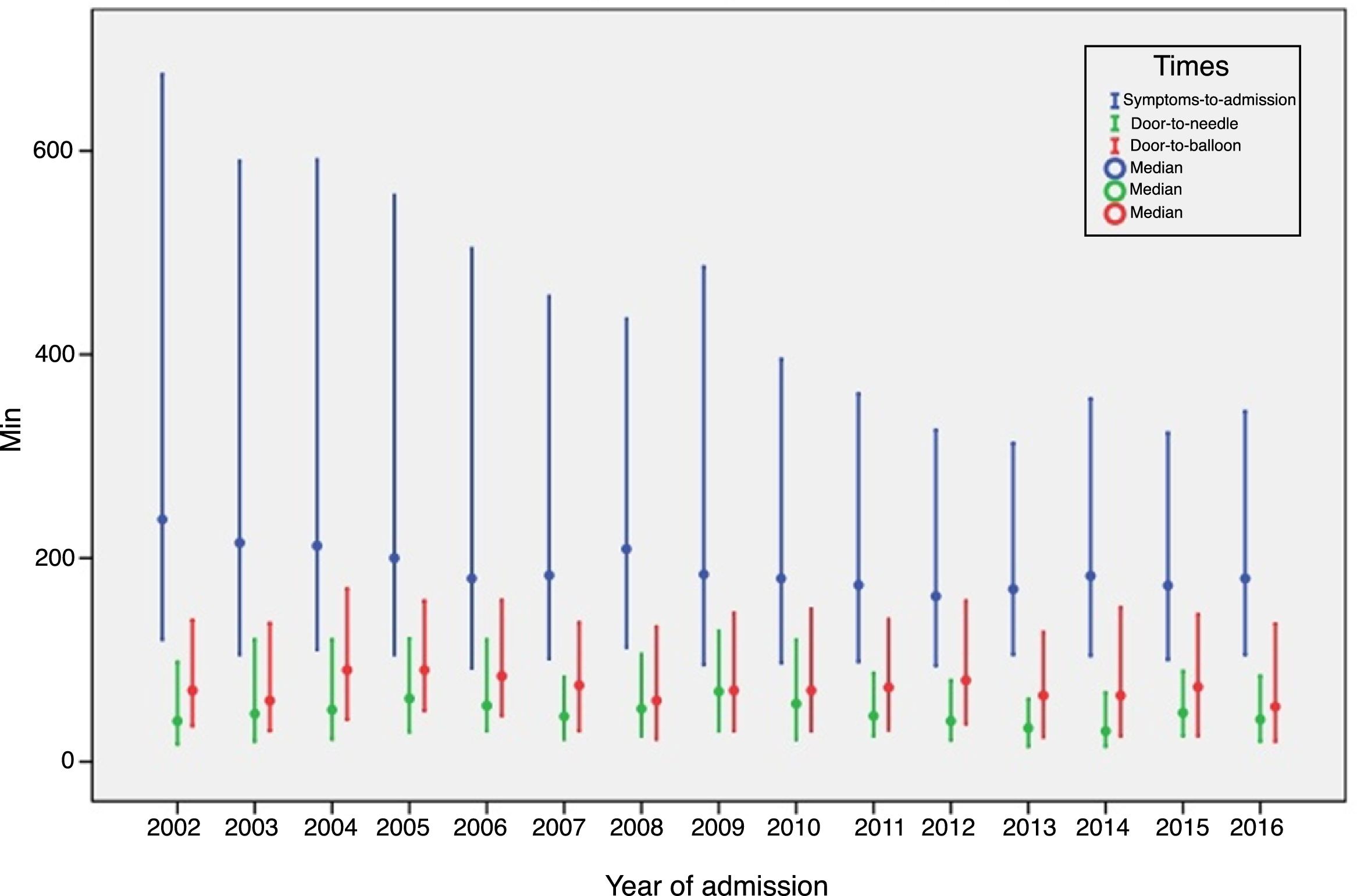
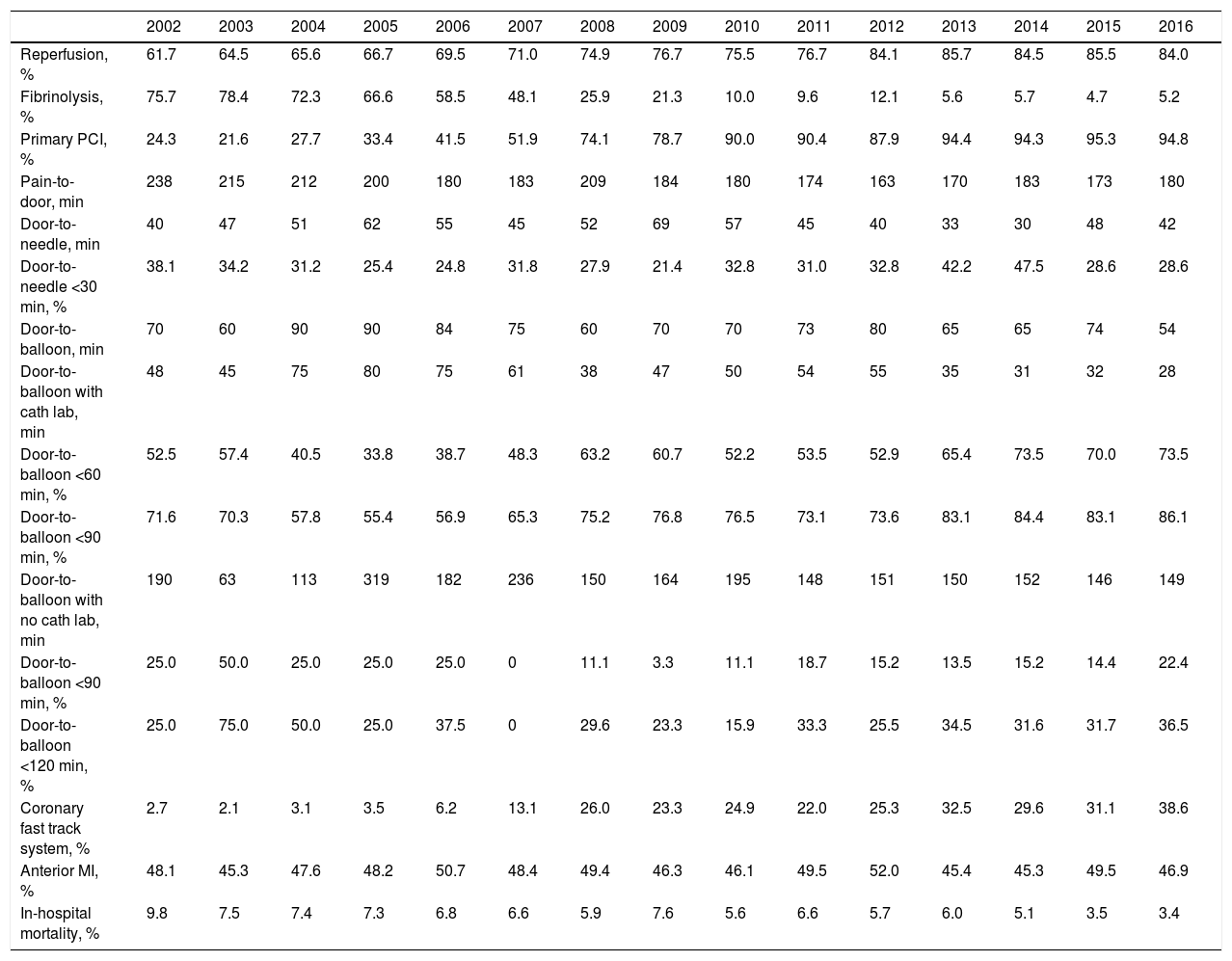
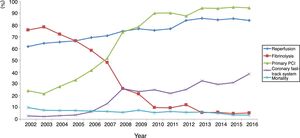
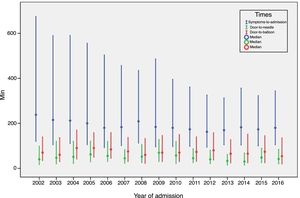
ST-elevation myocardial infarctionTreatment of STEMI underwent steady improvements over the years, especially in reperfusion rates (Table 4). The main reasons that reperfusion was not performed were late presentation (59.9% of cases), coronary anatomy (in 3% who did not undergo primary PCI) and contraindication for fibrinolysis (in 5% of fibrinolysis cases). The use of fibrinolysis decreased steadily (it is rarely used nowadays) and primary PCI was used in more cases from 2007 onward (Figure 3). In addition, use of the coronary fast-track system increased, accounting for over 35% of referrals in 2016. However, this progress was not accompanied by significant improvements in timings (symptoms-to-admission, door-to-needle and door-to-balloon), which have remained stable, especially in recent years (Figure 4). Compliance with recommended timings improved in centers with a catheterization laboratory, where the mean door-to-balloon time was <60 min in 73.5% of cases in 2016, up from 52.5% in 2002, and where in 2016 mean door-to-balloon time was <90 min in 86.1% of cases. For patients admitted to centers without catheterization laboratories (23.7% of those who underwent primary PCI), the timings varied considerably over the years; in 2016, only 14.6% achieved <60 min, 22.4% <90 min and 36.5% <120 min. It should be noted that the marked variability seen in the first years of the registry in hospitals without catheterization laboratories is related to the small number of patients treated at these centers. The median admission-to-ECG time was stable at 15-17 min, but with wide interquartile ranges, between 8 and 50 min in 2016.
Treatment of ST-elevation myocardial infarction.
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reperfusion, % | 61.7 | 64.5 | 65.6 | 66.7 | 69.5 | 71.0 | 74.9 | 76.7 | 75.5 | 76.7 | 84.1 | 85.7 | 84.5 | 85.5 | 84.0 |
| Fibrinolysis, % | 75.7 | 78.4 | 72.3 | 66.6 | 58.5 | 48.1 | 25.9 | 21.3 | 10.0 | 9.6 | 12.1 | 5.6 | 5.7 | 4.7 | 5.2 |
| Primary PCI, % | 24.3 | 21.6 | 27.7 | 33.4 | 41.5 | 51.9 | 74.1 | 78.7 | 90.0 | 90.4 | 87.9 | 94.4 | 94.3 | 95.3 | 94.8 |
| Pain-to-door, min | 238 | 215 | 212 | 200 | 180 | 183 | 209 | 184 | 180 | 174 | 163 | 170 | 183 | 173 | 180 |
| Door-to-needle, min | 40 | 47 | 51 | 62 | 55 | 45 | 52 | 69 | 57 | 45 | 40 | 33 | 30 | 48 | 42 |
| Door-to-needle <30 min, % | 38.1 | 34.2 | 31.2 | 25.4 | 24.8 | 31.8 | 27.9 | 21.4 | 32.8 | 31.0 | 32.8 | 42.2 | 47.5 | 28.6 | 28.6 |
| Door-to-balloon, min | 70 | 60 | 90 | 90 | 84 | 75 | 60 | 70 | 70 | 73 | 80 | 65 | 65 | 74 | 54 |
| Door-to-balloon with cath lab, min | 48 | 45 | 75 | 80 | 75 | 61 | 38 | 47 | 50 | 54 | 55 | 35 | 31 | 32 | 28 |
| Door-to-balloon <60 min, % | 52.5 | 57.4 | 40.5 | 33.8 | 38.7 | 48.3 | 63.2 | 60.7 | 52.2 | 53.5 | 52.9 | 65.4 | 73.5 | 70.0 | 73.5 |
| Door-to-balloon <90 min, % | 71.6 | 70.3 | 57.8 | 55.4 | 56.9 | 65.3 | 75.2 | 76.8 | 76.5 | 73.1 | 73.6 | 83.1 | 84.4 | 83.1 | 86.1 |
| Door-to-balloon with no cath lab, min | 190 | 63 | 113 | 319 | 182 | 236 | 150 | 164 | 195 | 148 | 151 | 150 | 152 | 146 | 149 |
| Door-to-balloon <90 min, % | 25.0 | 50.0 | 25.0 | 25.0 | 25.0 | 0 | 11.1 | 3.3 | 11.1 | 18.7 | 15.2 | 13.5 | 15.2 | 14.4 | 22.4 |
| Door-to-balloon <120 min, % | 25.0 | 75.0 | 50.0 | 25.0 | 37.5 | 0 | 29.6 | 23.3 | 15.9 | 33.3 | 25.5 | 34.5 | 31.6 | 31.7 | 36.5 |
| Coronary fast track system, % | 2.7 | 2.1 | 3.1 | 3.5 | 6.2 | 13.1 | 26.0 | 23.3 | 24.9 | 22.0 | 25.3 | 32.5 | 29.6 | 31.1 | 38.6 |
| Anterior MI, % | 48.1 | 45.3 | 47.6 | 48.2 | 50.7 | 48.4 | 49.4 | 46.3 | 46.1 | 49.5 | 52.0 | 45.4 | 45.3 | 49.5 | 46.9 |
| In-hospital mortality, % | 9.8 | 7.5 | 7.4 | 7.3 | 6.8 | 6.6 | 5.9 | 7.6 | 5.6 | 6.6 | 5.7 | 6.0 | 5.1 | 3.5 | 3.4 |
cath lab: catheterization laboratory; MI: myocardial infarction; PCI: percutaneous coronary intervention.
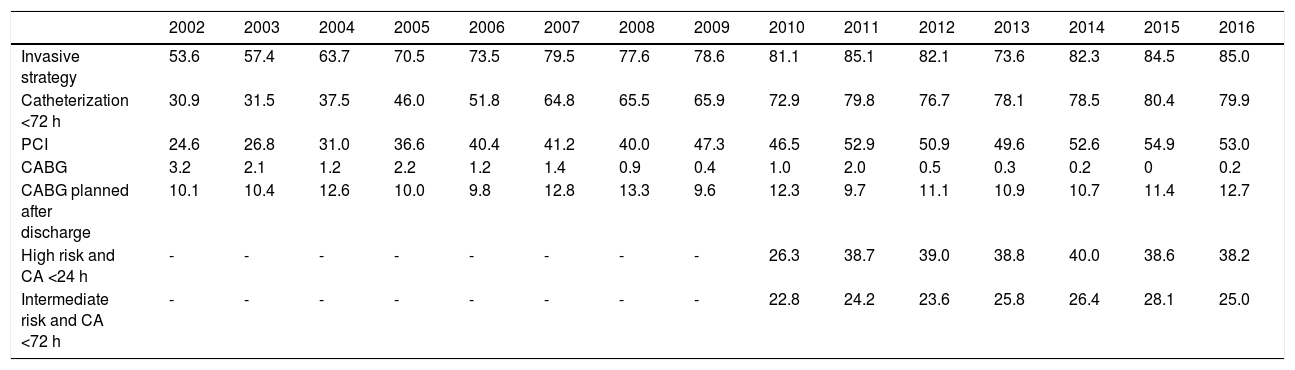
In non-ST-elevation acute coronary syndromes, both adoption of an invasive strategy (85% of cases in 2016) and rates of coronary angioplasty progressively increased (Table 5). By contrast, coronary artery bypass grafting was more often postponed to a later stage, often after discharge. Since 2010, it has been possible to analyze changes in compliance with the guidelines for timing of coronary angiography. It can be seen that although the percentage of high-risk individuals (GRACE score <140) undergoing angiography in the first 24 hours has increased, it was still only 38.2% in 2016, while of intermediate-risk patients (GRACE score 109-140), between 2010 and 2016 only around 25% underwent angiography within the recommended 72 hours.
Treatment of non-ST-elevation acute coronary syndrome (% of patients).
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Invasive strategy | 53.6 | 57.4 | 63.7 | 70.5 | 73.5 | 79.5 | 77.6 | 78.6 | 81.1 | 85.1 | 82.1 | 73.6 | 82.3 | 84.5 | 85.0 |
| Catheterization <72 h | 30.9 | 31.5 | 37.5 | 46.0 | 51.8 | 64.8 | 65.5 | 65.9 | 72.9 | 79.8 | 76.7 | 78.1 | 78.5 | 80.4 | 79.9 |
| PCI | 24.6 | 26.8 | 31.0 | 36.6 | 40.4 | 41.2 | 40.0 | 47.3 | 46.5 | 52.9 | 50.9 | 49.6 | 52.6 | 54.9 | 53.0 |
| CABG | 3.2 | 2.1 | 1.2 | 2.2 | 1.2 | 1.4 | 0.9 | 0.4 | 1.0 | 2.0 | 0.5 | 0.3 | 0.2 | 0 | 0.2 |
| CABG planned after discharge | 10.1 | 10.4 | 12.6 | 10.0 | 9.8 | 12.8 | 13.3 | 9.6 | 12.3 | 9.7 | 11.1 | 10.9 | 10.7 | 11.4 | 12.7 |
| High risk and CA <24 h | - | - | - | - | - | - | - | - | 26.3 | 38.7 | 39.0 | 38.8 | 40.0 | 38.6 | 38.2 |
| Intermediate risk and CA <72 h | - | - | - | - | - | - | - | - | 22.8 | 24.2 | 23.6 | 25.8 | 26.4 | 28.1 | 25.0 |
CA: coronary angiography; CABG: coronary artery bypass grafting; High risk: GRACE score >140; Intermediate risk: GRACE score 109-140; PCI: percutaneous coronary intervention.
Apart from heart failure, the most common complications were rhythm disorders, with new-onset AF in 5.2% of patients (decreasing from 6.6% in 2010 to 4.5% in 2016, p<0.001), and high-degree atrioventricular block in 3.1%. Resuscitated cardiac arrest occurred in 2.6% of patients, mechanical complications in 1.1%, stroke in 0.8% and major bleeding in 1.3%. Rates of all these complications were stable over the years, except for mechanical complications, which decreased from 1.5% in 2002 to 0.3% in 2016, and heart failure, which decreased from 35.6% to 15.3%. No changes were observed in major bleeding.
The use of pulmonary artery catheterization remained stable at around 0.1-0.3% of cases. There were also slight variations in the use of an intra-aortic balloon pump, which peaked in 2011 at 1.5% and fell thereafter to 0.3% in 2016. Use of a temporary pacemaker decreased from 3.0% in 2011 to 1.3% in 2016 (p<0.001) and implantation of a permanent pacemaker fell from 0.7% to 0.1% (p=0.011).
Left ventricular function was assessed in 93.7% of cases, increasing from 90% in 2002 to 98.7% in 2016, mostly by echocardiography. A significant percentage (39.0%) had impaired systolic function (ejection fraction <50%) and 7.7% had severe dysfunction (ejection fraction <30%), although these figures fell over the years (from 42.8% to 35.6% and from 12.2% to 3.2%, respectively, p<0.001).
A significant improvement should also be noted in the proportion of patients for whom cardiac rehabilitation was scheduled or planned at discharge, reaching 46.2% in 2016.
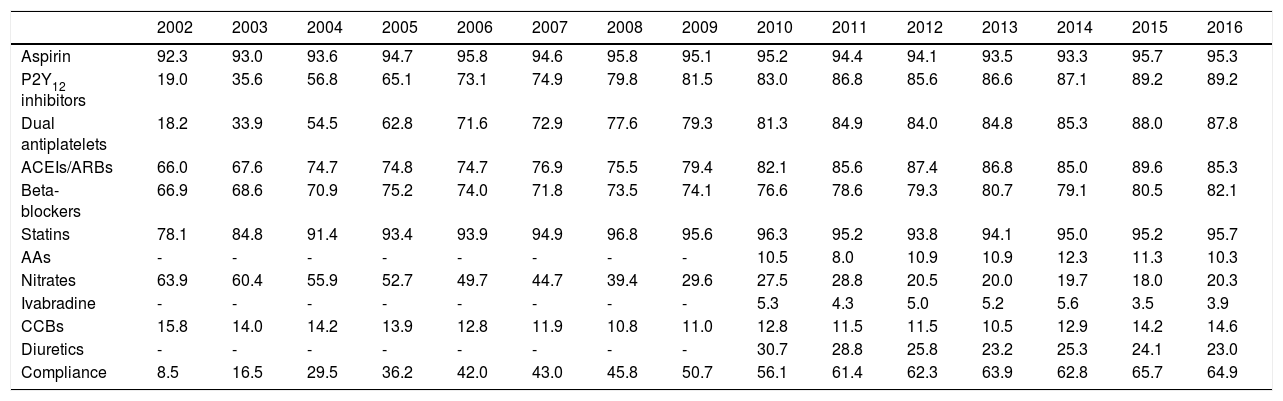
Therapy at dischargeAs for in-hospital medication, the use of antiplatelet therapy and other disease-modifying drugs at discharge underwent positive changes, particularly regarding statins, which in the second phase of the registry were being used in around 95% of patients who survived to discharge (Table 6). Among patients who are not on dual antiplatelet therapy, 72.5% were on one antiplatelet agent alone (aspirin in 61.7% of cases), 22.8% were on one antiplatelet agent and oral anticoagulation, and the rest received no antithrombotic therapy.
Medication at discharge in surviving patients (% of patients).
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin | 92.3 | 93.0 | 93.6 | 94.7 | 95.8 | 94.6 | 95.8 | 95.1 | 95.2 | 94.4 | 94.1 | 93.5 | 93.3 | 95.7 | 95.3 |
| P2Y12 inhibitors | 19.0 | 35.6 | 56.8 | 65.1 | 73.1 | 74.9 | 79.8 | 81.5 | 83.0 | 86.8 | 85.6 | 86.6 | 87.1 | 89.2 | 89.2 |
| Dual antiplatelets | 18.2 | 33.9 | 54.5 | 62.8 | 71.6 | 72.9 | 77.6 | 79.3 | 81.3 | 84.9 | 84.0 | 84.8 | 85.3 | 88.0 | 87.8 |
| ACEIs/ARBs | 66.0 | 67.6 | 74.7 | 74.8 | 74.7 | 76.9 | 75.5 | 79.4 | 82.1 | 85.6 | 87.4 | 86.8 | 85.0 | 89.6 | 85.3 |
| Beta-blockers | 66.9 | 68.6 | 70.9 | 75.2 | 74.0 | 71.8 | 73.5 | 74.1 | 76.6 | 78.6 | 79.3 | 80.7 | 79.1 | 80.5 | 82.1 |
| Statins | 78.1 | 84.8 | 91.4 | 93.4 | 93.9 | 94.9 | 96.8 | 95.6 | 96.3 | 95.2 | 93.8 | 94.1 | 95.0 | 95.2 | 95.7 |
| AAs | - | - | - | - | - | - | - | - | 10.5 | 8.0 | 10.9 | 10.9 | 12.3 | 11.3 | 10.3 |
| Nitrates | 63.9 | 60.4 | 55.9 | 52.7 | 49.7 | 44.7 | 39.4 | 29.6 | 27.5 | 28.8 | 20.5 | 20.0 | 19.7 | 18.0 | 20.3 |
| Ivabradine | - | - | - | - | - | - | - | - | 5.3 | 4.3 | 5.0 | 5.2 | 5.6 | 3.5 | 3.9 |
| CCBs | 15.8 | 14.0 | 14.2 | 13.9 | 12.8 | 11.9 | 10.8 | 11.0 | 12.8 | 11.5 | 11.5 | 10.5 | 12.9 | 14.2 | 14.6 |
| Diuretics | - | - | - | - | - | - | - | - | 30.7 | 28.8 | 25.8 | 23.2 | 25.3 | 24.1 | 23.0 |
| Compliance | 8.5 | 16.5 | 29.5 | 36.2 | 42.0 | 43.0 | 45.8 | 50.7 | 56.1 | 61.4 | 62.3 | 63.9 | 62.8 | 65.7 | 64.9 |
AAs: aldosterone antagonists; ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CCBs: calcium channel blockers.
Compliance with the recommendations in the guidelines for discharge medication (dual antiplatelet therapy, renin-angiotensin system blockers, beta-blockers and statins) improved significantly and remained at over 60% since 2011 (Figure 2).
Mortality in-hospital and during follow-upThere was a considerable reduction in all-cause in-hospital mortality over the study period (Figure 5). In the overall population, in-hospital mortality was 6.7% in 2002 and 2.5% in 2016, with similar progress in STEMI (9.8% to 3.4%, a reduction of 65%) and in non-ST-elevation MI (5.2% to 1.7%, a reduction of 67%).
Information on mortality during follow-up is limited and unreliable. Six-month follow-up data are available for only 44.6% of patients surviving to hospital discharge; in these, reported mortality was 5.2%.
DiscussionSince 2002, the ProACS registry has collected information on the presentation and diagnostic and therapeutic management of ACS in Portugal, in order to enable detailed assessment of changes over time and the impact of interventions, particularly through analysis of in-hospital mortality. It includes the full spectrum of ACS in real-world patients, unlike the selected participants included in clinical trials. The participation of a large number of centers means the registry is representative at a national level, although the fact that not all active centers participate and not all patients are included constitutes a significant limitation. Few national registries achieve this level of representativeness, which is only surpassed by those of Sweden (SWEDEHEART) and England and Wales (MINAP).8,9
In ProACS, data from evaluations of quality of treatment are sent periodically to all participating centers, to enable them to compare their center's approach with the national standard and thereby identify areas in which improvements in care could be made.
The clinical profile of patients changed little over the years, as did the proportion of ST-elevation ACS (close to 45%), as seen in other registries.8–13 There have been significant improvements, including better compliance with international guidelines, especially in STEMI, with high rates of reperfusion, which is now treated almost exclusively by primary PCI. Reperfusion rates are in line with those published in other countries, particularly in Europe; although primary PCI became the standard approach earlier (in 2004-2005) in most European countries,8–13 this occurred slightly later in the UK than in Portugal.13 This has resulted in progressive and consistent falls in in-hospital mortality from all forms of ACS. Unfortunately, time to reperfusion has not progressed sufficiently; delays are still too long, and there is considerable room for improvement, both through more campaigns aimed at the pre-hospital phase and by improving referral after first medical contact.14 In 2016, door-to-needle time was under 30 min in only 28.6% of patients and door-to-balloon time was under 90 min in only 63%. Reducing delays in reperfusion remains a priority in all programs for treatment of MI. In Portugal, there have been encouraging increases in referral via the coronary fast-track system, in which the National Institute for Emergency Medicine (INEM) has played an important role. The reduction in the proportion of patients in Killip class ≥2 or in cardiogenic shock may be related to this faster and more direct referral, as well as to earlier treatment of these patients.
In STEMI, delays at centers without interventional cardiology facilities are mainly due to difficulties with inter-hospital transportation. This is still a common problem, even in hospitals close to large urban centers, and can only be solved through organizational improvements, which need to be addressed locally as well as by the relevant authorities. Moreover, the first medical contact may be not with a hospital emergency department, but with primary or other health care facilities, which can delay not only the first ECG but also referral for reperfusion therapy. With regard to delays in ACS other than STEMI, particularly in high- or intermediate-risk patients, the causes are mainly related to inter- and intra-hospital issues. Among these is the lack of availability of places in cardiology wards, resulting in patients being held in emergency departments or in intermediate or intensive care units while awaiting coronary angiography, which is usually only performed in the cardiology department, as is risk stratification, which may also be delayed. Another issue that is beginning to pose problems in clinical practice is the increasingly widespread use of high-sensitivity troponin assays. This means that the proportion of patients with positive troponin is rising rapidly, and as troponin elevation is a high-risk criterion, this is leading to a marked increase in referrals for urgent coronary angiography. Measurement of troponin levels in this context needs to be examined in order to determine the most appropriate way in which they should be used, particularly what cutoff should be used for referral for coronary angiography within 24 hours.
The use of disease-modifying drugs, particularly dual antiplatelet therapy, renin-angiotensin system blockers, beta-blockers and statins, increased over time and prescription rates are similar to those reported in other registries, and in some cases even higher (especially of statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers). This marks an important advance in clinical application of the guidelines.7–11,15 GP IIb/IIIa inhibitors have been used less since 2002, coinciding with the routine use of dual antiplatelet therapy and the later introduction of more potent antiplatelet agents. However, GP IIb/IIIa inhibitors are still used in specific situations, which accounted for around 15% of cases in 2016. More frequent transport via INEM's coronary fast track system directly to the catheterization laboratory, where unfractionated heparin is the heparin of choice, may explain why this tended to replace enoxaparin after 2007, since heparins are not routinely administered in the pre-hospital phase. Prescription rates of dual antiplatelet therapy were high, even higher than in some other national registries, such as Norway, where only 68.1% of MI patients were discharged on dual antiplatelet therapy.
It is now standard procedure to assess left ventricular function, as recommended in the guidelines. However, pulmonary artery catheterization and intra-aortic balloon pump are now less commonly used, following publication of unfavorable or inconclusive studies on the routine use of these devices and consequent downgrading of the level of recommendation in the guidelines.16,17
The prevalence of AF, whether at admission or new-onset, was similar to that reported by other authors and has tended to decrease.18 In the era of primary PCI, the reported incidence of new-onset AF is 5-7.7%.18 Analysis of the characteristics of patients with AF or of the implications of this arrhythmia is beyond the scope of this work, but previous studies based on the ProACS registry showed that individuals with AF are older and mostly female, and have a high prevalence of hypertension, significant valve disease and heart failure, as well as being less likely to undergo coronary angiography or angioplasty, although there are no differences in terms of number of significantly diseased vessels. As in other studies, previously presented data based on ProACS showed that AF is a major cardiovascular risk factor for the occurrence of various complications, including heart failure, stroke, major bleeding and mortality.18–20
There was also a significant improvement in the rate of referral at discharge for scheduled or planned phase 2 cardiac rehabilitation programs, which rose from 18.8% in 2010 to 46.2% in 2016. Unfortunately, national surveys show that in practice, fewer than 10% of patients actually enroll in a rehabilitation program after discharge (unpublished data), and so there is still some way to go before Portugal reaches average European levels.
The ProACS registry has played an important role in clinical research on ACS conducted in Portugal. Since 2002, 199 studies based on data from the registry have been presented at Portuguese meetings, and 151 at international conferences, while six studies have been published in Portuguese journals and seven in foreign journals.
The number of continuous prospective international registries has increased since 2000, especially in the area of ACS. However, some are still based on regional rather than national data. Furthermore, no information has been published on the evolution over time of ACS care in Europe; the literature only contains recent data about registries in Asian or developing countries, which are not comparable. The Euro Heart Survey on Acute Coronary Syndromes presented its latest time-related data in 2004, the GRACE registry presented theirs in 2007 and SWEDEHEART (Sweden) and MINAP (England and Wales) issued their results up to 2010.7–13 Since then, no figures have been available to compare with our results, but up to 2010 our data were in line with those of other registries.
LimitationsThe data in the registry represent a significant proportion of the different geographical regions of Portugal. It is, however, not possible to guarantee that they accurately represent the entire population of the country. Centers participate in the registry on a voluntary basis, and so the consecutive inclusion of all patients admitted to participating centers cannot be ensured; furthermore, only those admitted to cardiology departments were included, meaning that an unknown number were admitted for ACS in other departments and therefore cannot be analyzed. It is our aim to involve the participation of a wider range of centers in order to improve the registry's national representativeness. Other national registries, such as those of England and Wales (100% of centers)9 and Sweden (95%)8 are more representative. Ideally, the ProACS registry will reach a similar level of representativeness to the National Registry of Interventional Cardiology, which currently includes over 95% of procedures and 100% of centers performing PCI in Portugal.21 Changes will have to be made in how centers participate in ProACS, taking into account obstacles to participation and ways in which the system could be improved.
It should also be noted that a significant proportion of fatal complications occur during the first few hours of ACS. Since only patients admitted to hospital are included, an unknown proportion of patients die before being admitted to participating centers and are therefore not analyzed.
There are also limitations relating to the variables collected in the registry. Infarct severity and extent cannot be analyzed on the basis of myocardial necrosis biomarkers, since these are measured using different laboratory methods, and thus direct comparisons are not possible. Accordingly, numerical values for troponin levels are not entered into the database, only whether or not troponin is elevated. Cardiac arrest rhythm is also not recorded, and so it is impossible to determine whether ventricular fibrillation or ventricular tachycardia was responsible, or whether the rhythm was primary or secondary, since entering the date and time of the event are optional.
Furthermore, there is limited information on patient follow-up, concerning which no conclusions can be drawn. This important limitation is due to the voluntary nature of centers’ participation and local logistical difficulties in collecting these data. It would have been particularly interesting to include information on therapy during follow-up, including whether patients were enrolled in a cardiac rehabilitation program following discharge.
A further limitation is the lack of internal and external auditing of ProACS data.
ConclusionsProACS is the largest active Portuguese registry and is the product of the ongoing endeavors of the centers involved. Using the data from patients at 45 centers, it has been possible to analyze the evolution over time of the characteristics of patients admitted with ACS, as well as their treatment and short-term prognosis. Clinical practice in Portugal is now closer to the guidelines and international practice, with more extensive use of disease-modifying drugs in hospital and at discharge, as well as of PCI. These improvements in compliance with the guidelines are to be welcomed, although there is still room for improvement in some aspects, particularly the persistence of long delays to reperfusion, for which there needs to be further efforts to increase public awareness and to streamline the national referral network. Nevertheless, the progressive reduction in in-hospital mortality is undoubtedly an incentive for all participants to strive for further improvements in the quality of care and hence the prognosis of these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Adriana Belo for providing the registry data and to Sandra Corker for her work on the administration of ProACS. We also thank all the participating centers whose patients were included in the registry:
Hospital de Braga, Hospital Senhora da Oliveira, Hospital São João Deus, Hospital São Pedro, Hospital Padre Américo, Hospital Pedro Hispano, Hospital São João, Hospital Geral Santo António, Hospital Vila Nova Gaia, Hospital Distrital Oliveira Azeméis, Hospital São Teotónio, Hospital Infante D. Pedro, Hospital Distrital Águeda, Hospital Sousa Martins, Centro Hospitalar Cova Beira, Hospitais Universidade Coimbra, Hospital Covões, Hospital Amato Lusitano, Hospital Leiria, Centro Hospitalar Médio-Tejo, Hospital Distrital Santarém, Hospital Reynaldo dos Santos, Hospital Dr. José Almeida, Hospital Curry Cabral, Hospital Fernando Fonseca, Hospital Militar Principal, Hospital Egas Moniz, Hospital São Francisco Xavier, Hospital Santa Cruz, Hospital Pulido Valente, Hospital Santa Maria (Cardiologia), Hospital Santa Maria (Medicina IC), Hospital Santa Maria (Medicina IA), Hospital Santa Marta, Hospital Garcia Orta, Hospital Nossa Senhora Rosário, Hospital Setúbal, Hospital Santa Luzia, Hospital do Espirito Santo (Évora), Hospital José Joaquim Fernandes, Hospital Barlavento Algarvio, Hospital de Faro, Hospital Espirito Santo (Angra Heroísmo), Hospital Distrital Horta, Hospital Divino Espirito Santo, Hospital Dr. Nélio Mendonça, Hospital CUF, Hospital Beatriz Ângelo
Please cite this article as: Timóteo AT, Mimoso J. Registo Nacional de Síndromes Coronárias Agudas: 15 anos de um registo prospetivo contínuo. Rev Port Cardiol. 2018;37:563–573.