Paravalvular leak (PVL) is a possible complication after prosthetic valve implantation. PVL can cause significant symptoms of congestive heart failure and/or hemolysis. Medical therapy is palliative and reoperation has a high mortality rate. Percutaneous transcatheter closure is a promising alternative for symptomatic patients at high surgical risk. We aim to review the efficacy and safety of percutaneous PVL closure in a consecutive series of patients referred to our center.
MethodsWe performed a retrospective analysis of clinical and technical procedural data of patients referred to our center for percutaneous PVL closure between January 2009 and November 2015.
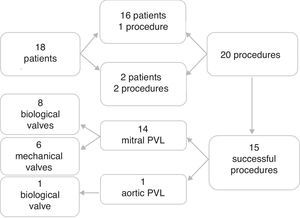
ResultsTwenty procedures were performed in 18 patients under general anesthesia and under transesophageal echocardiographic and radiographic guidance. Fourteen mitral PVLs were successfully treated in 13 patients and one aortic PVL in one patient. Most (eight) of the PVLs closed were in mitral bioprostheses. Two patients underwent a second intervention, which was technically successful in one. Technical success was achieved in 15 (75%) of the procedures. At discharge, median NYHA functional class decreased by one and hemolytic anemia decreased from seven cases (38.9%) to two (11.1%). Two patients had minor bleeding at the femoral vascular access site. Survival rates at six, 12 and 24 months were 77.8%, 77.8% and 61.1%, respectively.
ConclusionsIn our experience, percutaneous PVL closure was overall effective and safe. The procedure is complex and a second intervention may be necessary. Percutaneous PVL closure may be a feasible alternative for selected symptomatic patients at high surgical risk refractory to medical therapy.
As regurgitações paravalvulares (RPV) constituem uma complicação da cirurgia de substituição valvular. Em alguns casos podem surgir sintomas de insuficiência cardíaca congestiva e/ou hemólise. A terapêutica médica é paliativa e a reoperação associa-se a elevada taxa de mortalidade. O encerramento percutâneo poderá ser uma alternativa para doentes com elevado risco cirúrgico. Pretendemos rever a eficácia e segurança do encerramento percutâneo de RPV numa série de doentes consecutivamente referenciados ao nosso centro.
MétodosAnálise retrospetiva de dados clínicos e dos procedimentos efetuados em doentes referenciados para encerramento percutâneo de RPV, de janeiro de 2009 a novembro de 2015.
ResultadosVinte procedimentos foram efetuados em 18 doentes, sob anestesia geral, orientação radiológica e por ecocardiografia transesofágica. Foram tratadas 14 RPV mitrais com sucesso em 13 doentes e uma RPV aórtica. A maioria das RPV foram encerradas em biopróteses mitrais. Dois doentes foram submetidos a uma reintervenção, tecnicamente bem sucedida num deles. O sucesso técnico foi atingido em 15 (75%) dos procedimentos. À data de alta, a mediana de classe funcional NYHA diminuiu numa classe e a hemólise de sete (38,9%) para dois (11,1%) doentes. Dois doentes tiveram hemorragias minor nos locais dos acessos vasculares femorais. As taxas de sobrevivência aos seis, 12 e 24 meses foram 77,8, 77,8 e 61,1%, respetivamente.
ConclusõesA nossa experiência foi globalmente eficaz e segura. O procedimento é complexo e uma reintervenção pode ser necessária. Esta técnica poderá constituir uma alternativa para doentes selecionados, sintomáticos, de elevado risco cirúrgico e refratários à terapêutica médica.
Paravalvular leak (PVL) is an uncommon but potentially serious complication after prosthetic valve implantation. PVLs consist of an abnormal communication between two cardiac chambers adjacent to a prosthetic valve and manifest as a regurgitant jet originating between the outer margin of the prosthetic valve and the native tissue surrounding it. They may be due to abnormal pressure or traction forces on the prosthesis after surgery, and several factors are known to increase the risk of PVL, including annular calcification, infection, suturing technique, size and shape of the prosthetic implant and tissue friability. Early PVL is usually related to technical aspects of the surgical implant, whereas late PVL commonly results from suture dehiscence caused by endocarditis or the gradual resorption of incompletely debrided annular calcifications.1,2
The incidence of PVL is estimated to be 2-10% after surgical aortic valve replacement and 7-17% after mitral valve replacement. Most PVLs are asymptomatic, but in 1-5% of cases they have severe clinical consequences, with symptoms of congestive heart failure (CHF), hemolysis, or both.1,3
Until recently, surgery was the standard treatment, despite the high morbidity and mortality associated with reoperation. Alternatives to surgical closure of PVLs have been pursued over the years and in 1992 the first report on percutaneous PVL closure was presented, proving that the principles of percutaneous techniques to close intracardiac shunts could be translated to percutaneous PVL closure.1,2,4 Since then, percutaneous approaches to PVL closure have been developed as a less invasive strategy, through transseptal, apical left ventricular or retrograde arterial access, and a number of series have been published, with high rates of procedural success and favorable clinical outcomes.5
This study reports our experience with this technique, aiming to review the efficacy and safety of percutaneous device closure in a consecutive series of patients with clinically significant PVL referred to our center.
MethodsDefinitionsPVL was defined as a regurgitant jet originating between the outer margin of the prosthetic sewing ring and surrounding native tissues, demonstrated by Doppler echocardiography. CHF was defined as symptoms of heart failure consistent with New York Heart Association (NYHA) functional class of II or higher, and symptomatic hemolysis as hemolytic anemia (hemoglobin <13 g/dl in men or <12 g/dl in women, lactate dehydrogenase ≥600 mg/dl, or haptoglobin ≤10 mg/dl) requiring blood transfusion and/or erythropoietin injections, without any other source of blood loss.3
Technical success was defined as successful deployment of the closure device across the PVL without mechanical interference with the prosthesis or conversion to surgery. Clinical success was defined as an improvement of at least one NYHA functional class and/or an improvement in hemolysis, allowing the patient to become transfusion free.3
Patient populationBetween January 2009 and November 2015, 18 patients were referred to our Centre for possible percutaneous PVL closure. Patient clinical characteristics are shown in Table 1. The median age was 71 years (range 45-81) and 8 (44.4%) were male. Ten patients (55.6%) had one prosthetic valve, six (33.3%) had two and two patients (11.1%) had three prosthetic valves, in mitral, aortic and/or tricuspid position. The median time since surgical valve replacement was 47.5 months (range 1-1283 months). Most of the patients were hypertensive and had a history of paroxysmal or permanent atrial fibrillation. Five patients had a history of coronary artery disease and three had previously undergone coronary artery bypass grafting.
Patient characteristics.
| Male | 8 (44.4) |
| Age (years) | 71 (45-81) |
| Time since valvular replacement (months) | 47.5 (1-1283) |
| Number of prosthetic valves | 1 (1-3) |
| Comorbidities | |
| Hypertension | 9 (50.0) |
| Coronary artery disease | 5 (27.8) |
| Prior CABG | 3 (16.7) |
| Atrial fibrillation | 10 (55.6) |
| KDOQI stage ≥3 chronic kidney disease | 2 (11.2) |
| NYHA class | II (I-IV) |
| Hemolysis | 7 (38.9) |
Data are presented as number (percentage) or median (range). CABG: coronary artery bypass grafting; KDOQI: Kidney Disease Outcomes Quality Initiative; NYHA: New York Heart Association.
All patients referred for percutaneous PVL closure were symptomatic, with CHF and/or hemolysis, and were considered at high surgical risk after assessment by the local heart team. CHF alone was the indication for PVL closure in six patients (33.3%), hemolysis in three (16.7%) and both in five patients (27.8%); three patients underwent percutaneous PVL closure after a moderate to severe PVL had been documented after surgery and in one of the patients the indication was not specified. Written informed consent was obtained for every patient. The procedures were performed in the catheterization laboratory, under general anesthesia.
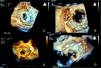
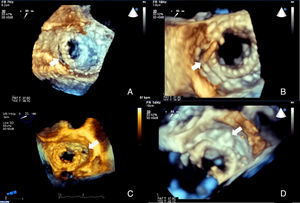
ImagingAll patients underwent pre-procedural transthoracic (TTE) and transesophageal (TEE) echocardiography for assessment of the morphology and function of the native and prosthetic heart valves. The degree of valvular regurgitation and/or stenosis was assessed by Doppler echocardiography, in accordance with the recommendations of the European Association of Cardiovascular Imaging.6,7 The size, location and shape of each PVL orifice was imaged, analyzed and measured (Figure 1).
Two-dimensional (2D) and real-time three-dimensional (3D) TEE, including live real-time, real-time 3D zoom and full volume acquisition, with or without color Doppler, were used in the planning and guidance of each procedure. The operator used the displayed images in order to select the approach and to guide the catheters and the device. Successful crossing of the defect was confirmed by 3D TEE (Figure 1).
Paravalvular leak closure techniqueA transseptal approach was used for the closure of mitral PVLs (95.0% [19] of procedures), crossing the mitral leak from the left atrium to the left ventricle, through an anterograde entry (not crossing the aortic valve). An arteriovenous loop was not used. A retrograde approach (from the ascending aorta to the left ventricle) was used for closure of aortic PVLs. A transapical approach was not adopted during this period. Amplatzer®-type devices (AGA Medical, Plymouth, Minnesota) were used for PVL closure.
Follow-upAt the end of the procedure all patients underwent echocardiographic study to assess the presence of residual leak and to exclude complications such as pericardial effusion, intracardiac thrombi or new intracardiac shunt. Clinical follow-up to assess NYHA functional class and need for blood transfusion was performed in all patients.
Statistical analysisClinical, echocardiographic and procedural data were obtained from retrospective review of patients’ records. The statistical analysis were performed using the SPSS statistical package (version 22.0, IBM SPSS, Chicago, Illinois). Continuous variables were reported as medians and range and categorical variables as numbers and percentage.
ResultsThere were 20 procedures in 18 patients with leaks in biological valve prostheses (50.0%) and in mechanical valve prostheses (50.0%) (Video 1). Two patients underwent two procedures, both of which failed due to inability to cross the leak with the guidewire. One had a PVL in a mechanical mitral valve, while the other had two PVLs in a mitral bioprosthesis in septal position, one successfully closed with an Amplatzer® Duct Occluder device and another with an Amplatzer™ Vascular Plug device five months later. This was the only patient to receive two devices. Most of the PVLs closed were in mitral bioprostheses (Figure 2). There were 14 mitral PVLs treated in 13 patients (Video 2) and one aortic PVL treated in one patient.
Study group profile. Eighteen patients were referred for percutaneous PVL closure at our center. Sixteen patients underwent a single procedure and two a second procedure, in a total of 20 procedures. Fifteen procedures were successful: 14 PVL closures in mitral prostheses (eight in biological and six in mechanical mitral valves) and one in a biological aortic prosthesis. PVL: paravalvular leak.
Technical success was achieved in 75% (15) of the procedures. PVL closure failed in five interventions: two cases due to inability to cross the leak with the guidewire and three cases due to interference with the mechanical function of the prosthetic valve. The Amplatzer™ Vascular Plug III was used in nine (50.0%) patients and the Amplatzer™ Duct Occluder in two (11.2%). The Amplatzer™ Vascular Plug II, Amplatzer™ Muscular VSD Occluder and Amplatzer™ PDA closure device were used in one patient (5.6%) each. No device was implanted in four patients (22.2%). Median fluoroscopy time was 40.8 min for all procedures, 43.5 min (range 16.8-92.6) for mitral PVL closure and 28.4 min for aortic PVL closure. Table 2 shows the results of the interventions.
Procedural outcomes.
| Total no. of procedures | 20 |
| Mitral PVLs to treat | 18 |
| Mitral PVLs treated | 13 (72.2) |
| Mitral PVLs requiring second procedure | 2 (11.1) |
| Aortic PVLs to treat | 1 |
| Aortic PVLs treated | 1 (100) |
| Approach | |
| Transseptal | 19 (95.0) |
| Retrograde (transaortic) | 1 (5.0) |
| Fluoroscopy time (min) | |
| Mitral PVL | 43.5 (16.8-92.6) |
| Aortic PVL | 28.4 |
| Procedural failure | 5 (25.0) |
| Inability to cross with guidewire | 2 (10.0) |
| Interference with function of prosthesis | 3 (15.0) |
| Complications | |
| Bleeding through vascular access | 2 (10.0) |
PVL: paravalvular leak. Data are presented as number (percentage) or median (range).
Technical success was achieved in 75% (15) of the procedures (Video 3). Median NYHA functional class decreased by one after the procedure. Cases of hemolytic anemia decreased from seven (38.9%) pre-procedure to two (11.1%) at discharge. Two patients had minor bleeding at the femoral vascular access site. There were no arterial dissections, device embolizations, conversion to surgery or deaths related to the procedure. At six, 12 and 24 months, NYHA functional class and rate of hemolytic anemia remained stable. Four patients died during follow-up due to refractory CHF (Table 3). Survival rates at six, 12 and 24 months were 77.8%, 77.8% and 61.1%, respectively.
Mortality at follow-up.
| Patient | Gender | Age | Type of PVL | Location of leak | Successful PVL closure | Cause of death | Time after procedure (months) |
|---|---|---|---|---|---|---|---|
| 1 | Female | 75 | Biological mitral valve | Anterolateral | Yes | Cardiogenic shock | 0a |
| 2 | Female | 71 | Biological mitral valve | Anterolateral | No | CHF | 2 |
| 3 | Male | 70 | Mechanical mitral valve | Anteroseptal | Yes | CHF | 20 |
| 4 | Female | 70 | Mechanical mitral valve | Medial | No | CHF | 28 |
CHF: congestive heart failure.
Small PVLs can be found on echocardiography in almost half of patients with prosthetic heart valves. In up to 5% of these patients, a larger leak with clinical significance may be apparent, most often associated with mitral valves, less often with aortic and rarely with tricuspid or pulmonary valves. Whether bioprostheses are associated with a greater risk of PVL than mechanical valves is still a matter of debate.8
Clinical presentation may occur soon after surgery or years later, as documented by our experience. Symptoms may be due to the degree of regurgitation, leading to low effective cardiac output, CHF and/or hemolytic anemia, which arises from fragmentation of red blood cells in the high shear stress of the regurgitant jet. Primary or secondary infective endocarditis can also occur.8
Spontaneous closure of PVLs is rare and medical treatment is largely palliative. Surgical correction has been the gold standard for treatment of PVL but is usually reserved for severely symptomatic patients and/or those with progressive cardiac dysfunction. The surgical mortality rate can be as high as 13% after the first procedure, in part due to the age and comorbidities of these patients, and in many symptomatic patients reoperation carries excessive risk and is usually not an option.3,8
Our experience demonstrates that percutaneous PVL closure can be a feasible treatment alternative in patients who are not candidates for surgery. As previously reported, percutaneous closure of PVL still faces several issues, particularly the lack of a specific device designed to treat PVL and hence off-label use of available devices; difficulty in crossing the leak due to the position or anatomical configuration of the defects, which can be serpiginous and/or rigid due to calcification of the annulus or to the valve annulus itself; and interference of the device with the function of the prosthetic valve, making it impossible to release the device.3,8 The operator's experience is crucial to the success of the intervention and may have accounted for our initial difficulties with this technique. The type of approach may also affect the final result, and a transapical approach may offer an alternative option in cases where transseptal or retrograde approaches are not feasible. The choice of device was at the discretion of the operator, based on echocardiographic and radiographic interpretation.
Procedural and clinical success as well as complication rates were acceptable for these 18 high surgical risk patients. A team approach, including transesophageal echocardiography specialists, interventional and general cardiologists and anesthesiologists, facilitated these outcomes. Such interventions are lengthy, as demonstrated by the relatively long fluoroscopy times, and can be challenging and technically demanding; close cooperation between team members in preprocedural planning and during and after the intervention are therefore essential.
Study limitations and future directionsThis was a retrospective study, subject to the limitations of this type of study. In addition, the relatively low number of patients included limits generalization of these results to a broader population.
In the future, the inclusion of a larger number of patients and of procedures will be necessary in order to determine the predictors of technical success and of short- and long-term morbidity and mortality. The development of specific and more adaptive devices that can conform to a variety of shapes of these defects and the use of multimodality imaging in the planning and guidance of interventions is necessary in order to further improve the results of the technique.
ConclusionsOur experience of PVL closure in a predominantly elderly, high-risk population was favorable in terms of technical success, clinical improvement and safety. The procedure is complex and a second intervention may be necessary.
Percutaneous closure of PVLs may be a feasible and valid alternative treatment strategy for selected symptomatic patients refractory to medical therapy and at high surgical risk. A collaborative effort between a skilled interventional team can result in successful outcomes, apparently with lower morbidity and mortality rates in comparison to surgical series.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data is showed in this article.
Right to privacy and informed consentThe authors declare that no patient data is showed in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.