Our goal was to assess usefulness of Pattern Matching Filter (PMF) software combined with the PentaRay catheter for complex premature ventricular contraction (PVC) ablation.
MethodsA prospective observational study of consecutive patients referred for complex PVC ablation at our tertiary center from January to September 2018. Patients underwent ablation using a pre-specified mapping strategy with the PMF and the PentaRay catheter (PVCs with ≥97% correlation with the template morphology were collected). Procedural endpoints and acute and 12-months success rates were assessed and compared to a retrospective cohort of patients who also underwent a complex PVC ablation with standard activation mapping performed with a Thermocool SmartTouch catheter.
ResultsDuring the nine-month enrollment period, seven patients fulfilled our inclusion criteria, while there were four patients in the control group. Patients treated with the PMF and PentaRay had a fivefold number of points acquired (507±213 vs. 90±62) and a halved procedure time (67±42 vs.130±54 min), required a shorter radiofrequency ablation time (294±112 vs.706±613 sec) and had a higher overall success rate (100% vs.75%) when compared to the standard approach. No major complications occurred in either group.
ConclusionsIn this first study assessing the combined use of the PentaRay catheter and the PMF for complex PVCs ablation, we demonstrate how this approach can improve the level of detail, accuracy and reliability of the activation map, while reducing the number of radiofrequency applications and procedural time. Further studies are warranted to confirm whether this approach can lead to improved outcomes.
Avaliar a utilidade da abordagem conjunta do softwarePattern Matching Filter (PMF) com o cateter PentaRay na ablação de extrassístole ventricular (ESV) complexa.
MétodosEstudo observacional prospetivo de doentes consecutivos referenciados para ablação de ESV complexa (re-dos ou ESV esquerda) de janeiro a setembro de 2018. Os pacientes foram submetidos a uma estratégia de mapeamento predefinida com o PMF e o cateter PentaRay (ESV com ≥97% de correlação com a morfologia definida). Os objetivos primários, bem como o sucesso agudo e a 12 meses, foram avaliados e comparados com uma coorte retrospetiva de pacientes também sujeitos a ablação de ESV complexa mas com um mapa de ativação convencional realizado com o cateter de ablação Thermocool SmartTouch.
ResultadosDurante os nove meses foram incluídos sete pacientes que preencheram os critérios e quatro foram no grupo controlo. Os pacientes tratados com o PMF e o cateter PentaRay tiveram cinco vezes mais pontos adquiridos (507±213 versus 90±62) e metade do tempo de procedimento (67±42 versus 130±54 min), apresentaram menor tempo de ablação (294±112 versus 706±613 sec) e uma maior taxa de sucesso (100% versus 75%) quando comparados com a abordagem convencional. Não se verificaram complicações em ambos os grupos.
ConclusõesNeste primeiro estudo a avaliar a abordagem conjunta do cateter PentaRay com o PMF para a ablação de ESV complexa, demonstramos que esta abordagem melhora o detalhe, a precisão e a fiabilidade do mapa de ativação, ao mesmo tempo que reduz o tempo de radiofrequência e do procedimento. Estudos adicionais serão necessários para confirmar se esta abordagem melhora o sucesso.
Catheter ablation is an established treatment for premature ventricular contractions (PVC), having demonstrated high efficacy and safety in several studies.1,2 However, when PVCs arise from outside the right ventricular outflow tract (RVOT), such as the left ventricular outflow tract (LVOT), aortic cusps, papillary muscles or the epicardial surface, the success rate is lower.2,3
Three-dimensional (3D) electroanatomic mapping systems have become an essential tool for mapping complex arrhythmias. More recently, new software called Pattern Matching Filter (PMF) (Biosense Webster), which enables a correlation between the predefined template morphology and the real time beat to be made, has been proposed to improve ablation outcomes.
Moreover, multielectrode catheters with smaller electrodes and closer interelectrode spacing have already demonstrated their advantage when mapping atrial or ventricular scars.3,4
The purpose of the current case series was to investigate the usefulness of the new software PMF in addition to the PentaRay multielectrode mapping catheter for complex PCV ablation.
MethodsStudy design and settingProspective observational study of consecutive patients with frequent symptomatic PVCs referred for catheter ablation at our center from January to September 2018. These patients underwent ablation using a pre-specified mapping strategy with the PMF software and the PentaRay catheter. Procedural endpoints and acute and 12-month follow-up results were assessed and compared to a 12-month retrospective cohort of patients who underwent complex PVC ablation with standard activation mapping performed with the use of the Thermocool SmartTouch irrigated tip contact force-sensing ablation catheter (ST - Biosense Webster).
All patients provided written informed consent and the study was approved by the local institutional ethics committee.
Patient eligibility criteriaPatients from both the study and control groups were eligible for inclusion in the study if they presented at least one criteria from group A plus at least one from group B. Group A criteria included: i) symptomatic drug-refractory PVCs, or ii) a decline in left ventricular ejection fraction (LVEF) due to frequent PVCs. Group B criteria included: i) a previous history of PVC ablation, or ii) a left-sided PVC. All patients underwent evaluation for any underlying structural heart disease, and major coronary artery disease had to be ruled out by cardiac angiography or stress testing in cases of LVEF dysfunction.
Exclusion criteria were as follows: presence of structural heart disease (ischemic heart disease, significant valvular disease or genetic or infiltrative cardiomyopathy), pregnancy and any clinical contraindication for the ablation procedure.
Ablation procedureAll procedures in both the prospective and retrospective groups were performed by the same primary operator. They were conducted under local anesthesia and all anti-arrhythmic drugs (AAD) stopped at least five half-lives before the procedure. For left-sided procedures, a retrograde approach was first choice, but access via transseptal puncture was also obtained whenever necessary. Intravenous heparin was administered to maintain an activated clotting time ≥300 seconds for left-sided procedures.
Anatomical mapping data was collected using a 3D mapping system (CARTO3®, Biosense Webster) and respiratory noise was excluded (AccuResp Module, Biosense Webster). Activation mapping was performed using a high-density system (Confidense® Module, Biosense Webster) and signal annotation conducted using Wavefront activation software (Biosense Webster), which analyses and integrates bipolar signals and the corresponding negative onset of unipolar signal.5
The use of intracardiac echocardiography was left to the physician's discretion.
Ablation was performed using a ST ablation catheter, in power-controlled mode with temperature limited to 43°C and saline irrigation speed of 17 mL/min. The power setting was 20 W and could be titrated up to 40 W, depending on the region being targeted.
When the ectopic focus was located in the coronary cusps, coronary angiography (CA) was routinely performed. If the focus was at a safe distance (>6 mm)2 from the coronary artery, radiofrequency (RF) was delivered. After ablation, CA was performed again in order to exclude any damage to the coronary arteries. When ablation was performed beneath the coronary cusps, CA was not required given the probable distance from the coronary arteries.
After ablation, patients were monitored for at least 30 minutes in de novo cases and 60 minutes in redo procedures to avoid recurrence.
All AAD were discontinued after successful procedures.
Systematic transthoracic echocardiography was performed before discharge to exclude aortic valve damage or pericardial effusion. A 24-h Holter was also performed before discharge.
Pattern Matching Filter softwareThis new software was released with version 6 of the CARTO3® system. It allows the acquisition of local activation time (LAT) points based on real time comparison of the 12-lead electrocardiogram (ECG) morphology with a morphology of interest (template). Essentially, this software enables a correlation to be made between the predefined morphology and every real time beat.
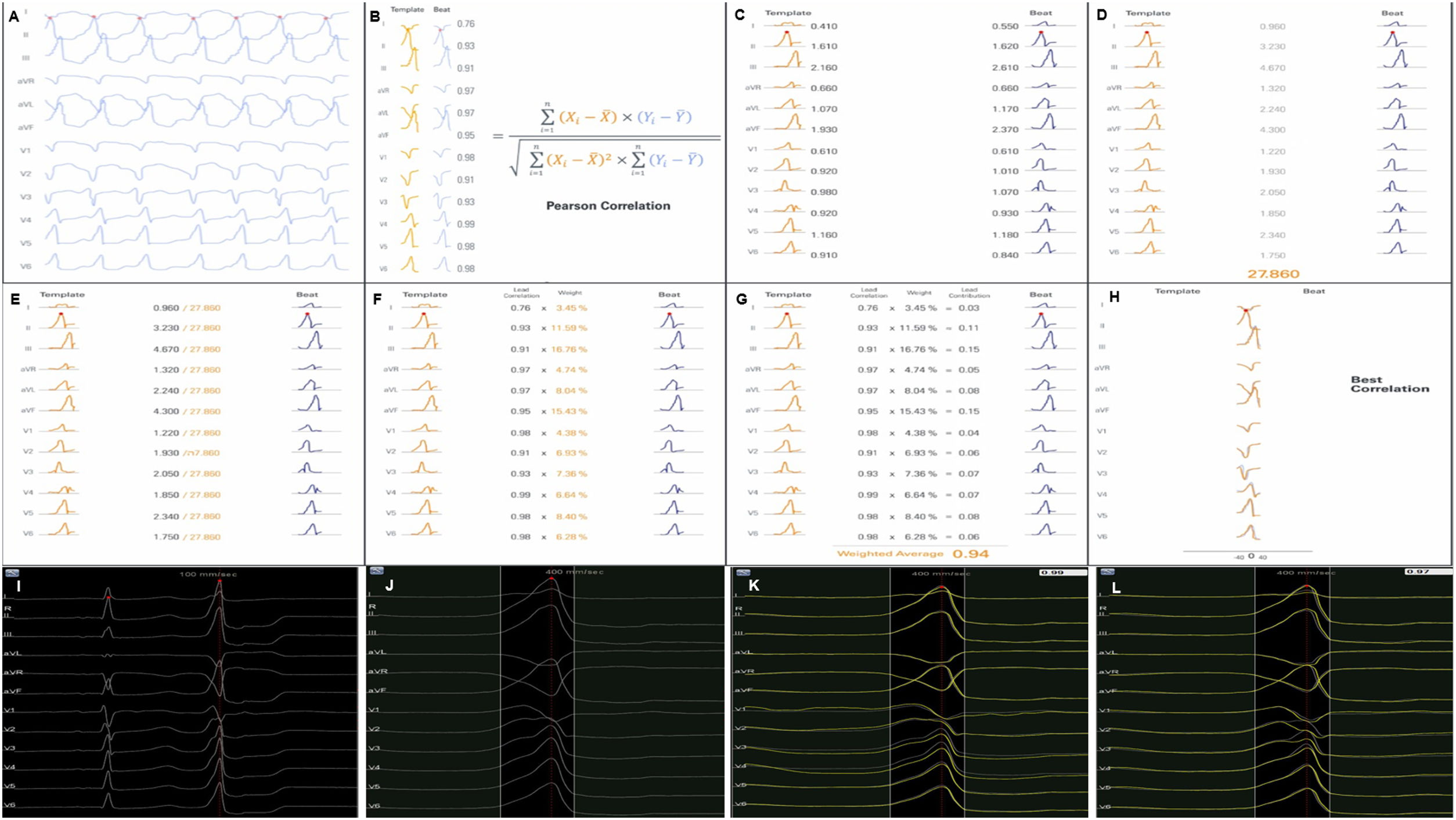
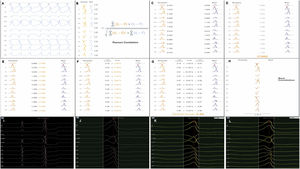
Each activation point collected with the mapping catheter must follow some intrinsic steps before validation (Figure 1A-H): (A) definition of the pattern – selection of the template morphology to be mapped and adjustment of the window of interest (WOI); (B) single channel correlation – the fit between the template and the current beat is calculated for each of the 12 leads using Pearson correlation formula; calculation of overall weighted – (C) in this step the absolute voltage is measured for each lead (for the template and for the beat); (D) lead correlation – the total sum of each pair of leads (template and beat) is calculated and then (E) divided by the total sum of all leads weight; (F) lead contribution – the lead correlation is multiplied by the weighted average and the result (G) is the final weighted average score; (H) phase shift of ±40 ms – this step takes into consideration a possible offset between reference annotations in beats with similar morphology. Finally, the Pattern Matching percentage is compared to the threshold – if the matching percentage is equal to or higher than the threshold, the beat is considered to represent the template morphology and is incorporated into the activation map.
(A-H) Pattern Matching Filter (PMF) algorithm workflow (as described in technical presentation for health care professionals; with permission from Biosense Webster). (I-L) Utilization of the PMF software in a patient with premature ventricular contraction arising from the left coronary cusp. I. Selection of the premature ventricular contraction as the template morphology to be mapped. J. Window of interest (WOI) adjustment in order to include only the QRS complex. K. A 95% minimum threshold was selected and during continuous mapping, each premature ventricular contraction and its respective correlation were visualized. L. Since the vast majority of premature ventricular contraction presented a correlation of between 97 and 99%, WOI was adjusted and the minimum threshold was set at 97%. After all the steps were performed, we started collecting geometry and activation points with the PentaRay catheter.
At the beginning of the procedure, while monitoring the patient, the culprit PVC was quickly acquired and defined as the template morphology to be mapped (Figure 1I-L). The morphology of the reference channel in sinus rhythm and in tachycardia should be similar to avoid missing beats. The WOI was adjusted to include only the QRS complex. Thereafter, in the Pattern Matching box we selected the pattern to be mapped and a minimum of 95% correlation. We were then able visualize, in continuous mapping, the correlation of the selected template to the real time PVCs and adjust the WOI again, if necessary. Also, we increased the threshold in all cases to at least 97%. After all these steps were performed, we started our mapping process with the PentaRay catheter and visualized which beats passed or failed in the selected template morphology.
The ventricular geometry, outflow tract or aortic cusps were collected with the PentaRay catheter and, when available, integrated with a CT reconstruction of the outflow tract (CartoMerge, Biosense Webster). The PentaRay has 1-mm electrodes with interelectrode spacings of 2-6-2 mm, in order to provide more detailed electrogram information. To avoid using extrapolation from ECG Wilson's triangle and noise, we placed a hexapolar catheter (Biosense Webster) on the right ventricle to obtain a clean unipolar signal since this catheter has a specific electrode placed in the inferior vena cava.
Our initial approach consisted of collecting only anatomical points with fast anatomical mapping (FAM). After defining the geometry, we started collecting LAT points. However, if at the beginning of the procedure the patient presented only rare PVCs, geometry and activation maps were performed simultaneously. If very few or no PVCs were observed at baseline, intravenous administration of isoproterenol or programmed ventricular stimulation was performed.
Only PVCs selected by the PMF – exceeding the minimum threshold (position stability=3 mm, maximum density and tissue contact selected) – were added to the LAT map and no LAT point was edited after automatic annotation. Once the focus was well defined, we shifted to an ST ablation catheter.
Control group patients (local activation time with ablation catheter)The approach was similar to the aforementioned, but the whole anatomy was constructed with the Thermocool SmartTouch catheter, which has a 3.5-mm electrode tip with interelectrode spacings of 1-6-2 mm. After defining the geometry with FAM, we started collecting LAT points. Points were also acquired automatically (position stability=3 mm, maximum density and a minimum tissue contact of 3 g), however each point required manual confirmation before validation and incorporation into the activation map.
Study endpointsThe primary endpoint of this study was the utility of the combined use of PMF with PentaRay catheter during the procedure, assessed by the number of activation points acquired, total RF time to eliminate PVC and overall procedure time.
Secondary endpoints included acute and 12-month effectiveness and safety of the ablation procedure. Acute success was defined as the complete abolition of the clinical ventricular arrhythmia at the end of a 30-minute waiting period (or 60 minutes for re-dos). Follow-up effectiveness was defined by a reduction in PVC burden of at least 80%6 and safety endpoints included any major complications (vascular injury requiring transfusion or vascular surgery, cardiac tamponade, stroke or other systemic emboli, and damage to the aortic valve or coronary arteries).
Follow-upAfter the index procedure, patients were routinely seen at three and 12-months. Data collected during follow-up included a 12-lead ECG and a 24-h Holter at each visit. In case of previous LV dysfunction, assessment of LVEF by echocardiography was also performed to confirm an improvement in LVEF.
ResultsDuring the nine-month enrollment period, a total of seven patients fulfilled our inclusion criteria and were treated with the combined use of the PMF software and the PentaRay catheter. The control group included four patients who underwent conventional mapping.
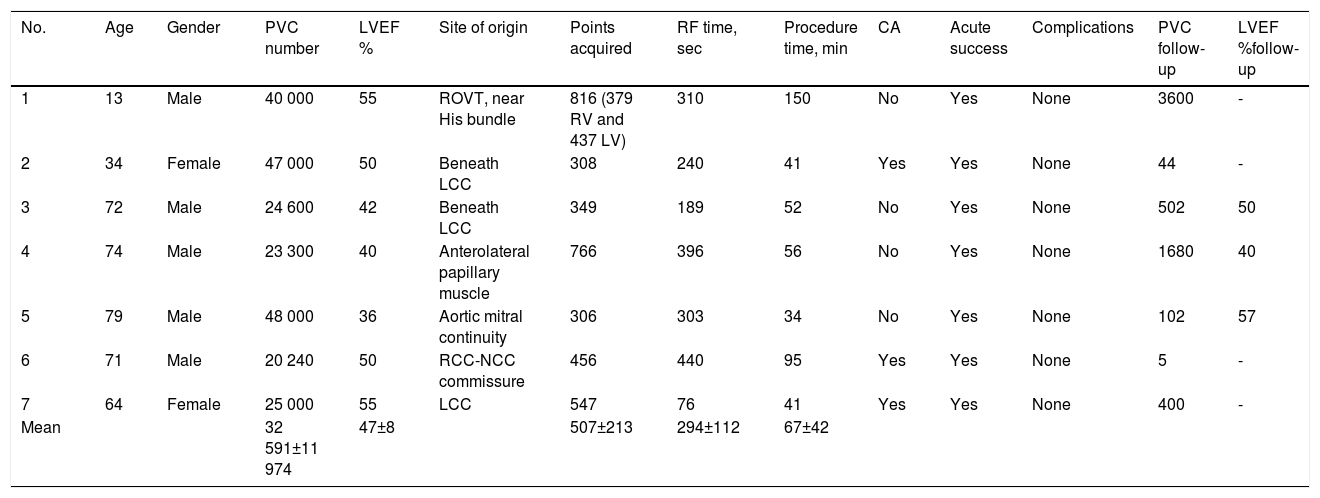
Study group (Pattern Matching Filter with PentaRay catheter)The seven patients from the PMF group are described in Table 1 – one was a 13-year old male undergoing a redo ablation for RVOT PVC, while the other six patients had PVCs arising from the LV (five from the LVOT and one from the anterolateral papillary muscle). All seven patients had a high PVC burden (>32 000 PVC/24 h), with multiple and long episodes of bigeminy in all of them. The mean LVEF was 47±8% and three patients had LVEF<45%.
Procedure data from the Pattern Matching Filter software with PentaRay catheter.
| No. | Age | Gender | PVC number | LVEF % | Site of origin | Points acquired | RF time, sec | Procedure time, min | CA | Acute success | Complications | PVC follow-up | LVEF %follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Male | 40 000 | 55 | ROVT, near His bundle | 816 (379 RV and 437 LV) | 310 | 150 | No | Yes | None | 3600 | - |
| 2 | 34 | Female | 47 000 | 50 | Beneath LCC | 308 | 240 | 41 | Yes | Yes | None | 44 | - |
| 3 | 72 | Male | 24 600 | 42 | Beneath LCC | 349 | 189 | 52 | No | Yes | None | 502 | 50 |
| 4 | 74 | Male | 23 300 | 40 | Anterolateral papillary muscle | 766 | 396 | 56 | No | Yes | None | 1680 | 40 |
| 5 | 79 | Male | 48 000 | 36 | Aortic mitral continuity | 306 | 303 | 34 | No | Yes | None | 102 | 57 |
| 6 | 71 | Male | 20 240 | 50 | RCC-NCC commissure | 456 | 440 | 95 | Yes | Yes | None | 5 | - |
| 7 | 64 | Female | 25 000 | 55 | LCC | 547 | 76 | 41 | Yes | Yes | None | 400 | - |
| Mean | 32 591±11 974 | 47±8 | 507±213 | 294±112 | 67±42 |
CA: coronary angiography; LCC: left coronary cusp; LVEF: left ventricular ejection fraction; NCC: non-coronary cusp; PVC: premature ventricular contraction; RCC: right coronary cusp; RF: radiofrequency; RVOT: right ventricular outflow tract.
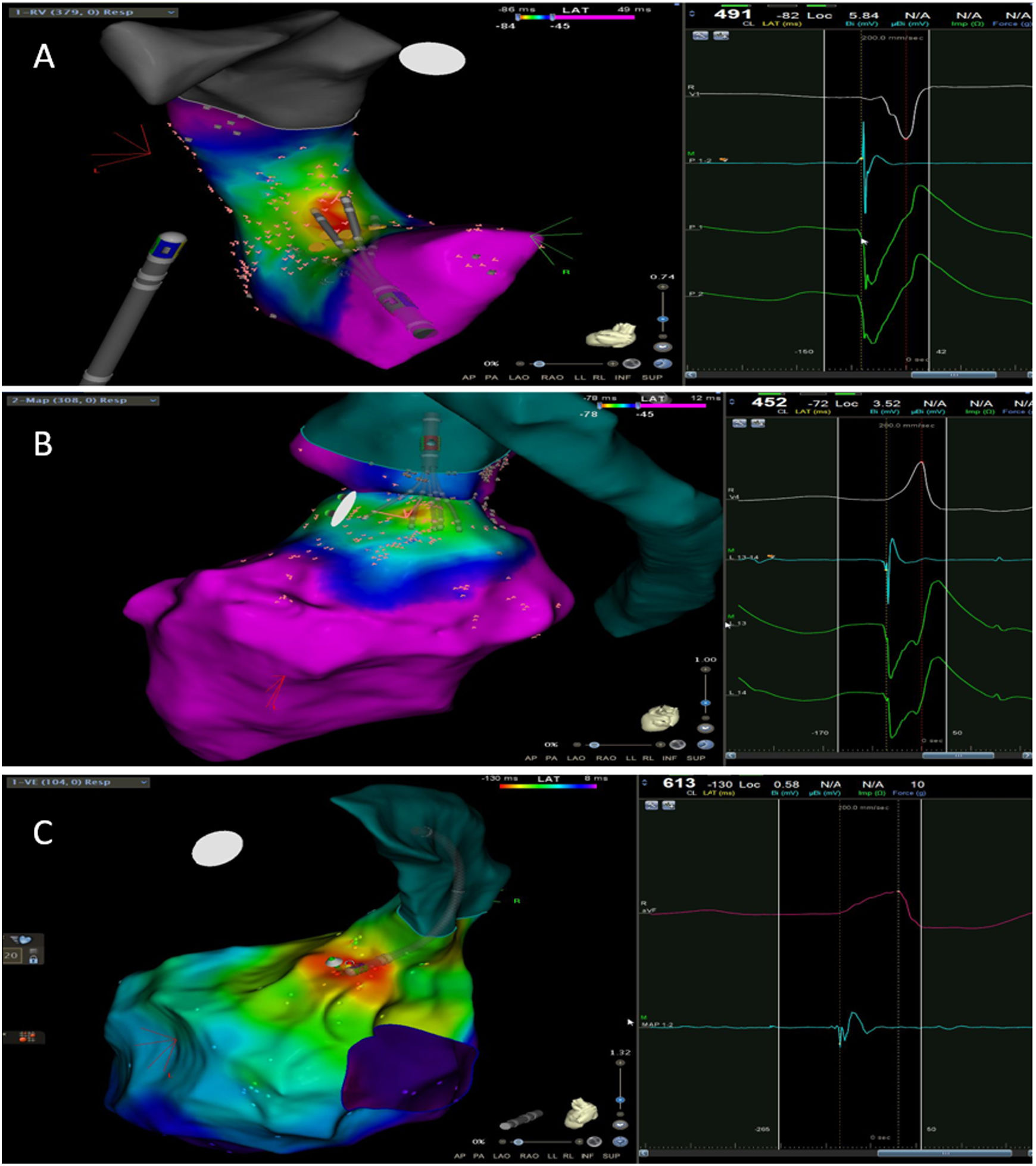
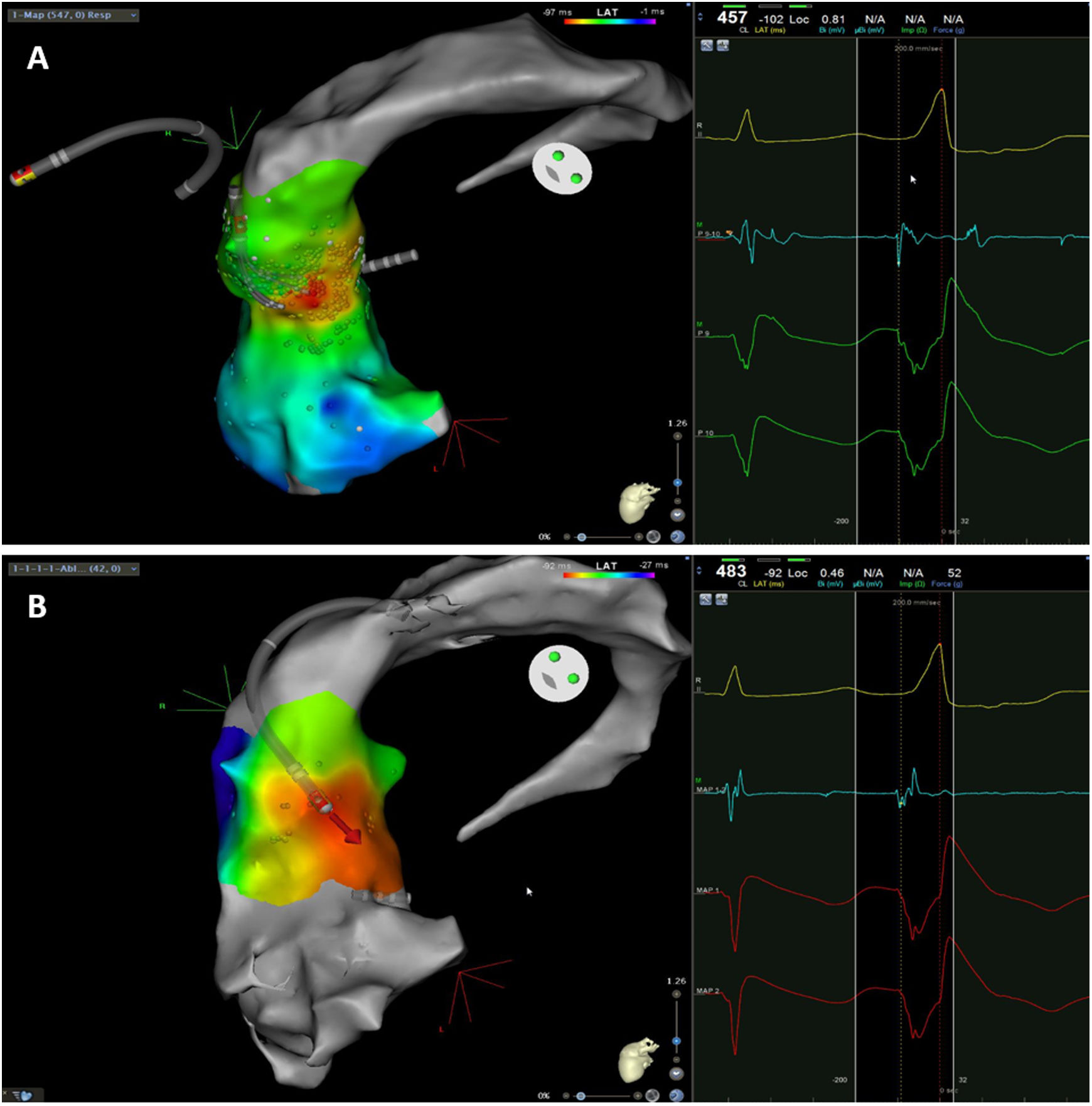
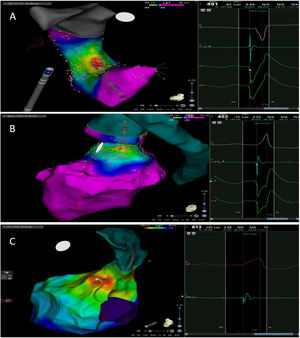
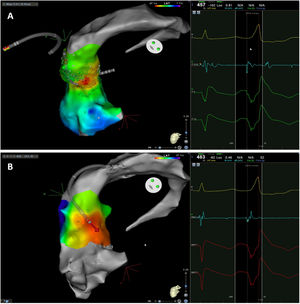
The mean number of points acquired with the PentaRay catheter was 507±213 before ablation during 15±10 min of mapping. In the first patient, who was undergoing a redo ablation of a PVC arising near the His bundle, mapping was carried out with the PentaRay in both the RVOT and the coronary cusps, particularly the non-coronary cusp (NCC) (Figure 2A). However, since the earliest activation site was located in the RVOT, with an optimal unipolar signal, ablation was performed there. In the other patients, the earliest LAT was located in the left coronary cusp (LCC) (n=3) – one in the LCC (Figure 3) and two beneath (Figure 2B), the papillary muscle (n=1), the aortic mitral continuity (AMC) (n=1) and the right (RCC)-NCC commissure (n=1). Median procedure and RF ablation times were 67±42 min and 294±112 s, respectively. Acute success was achieved in all patients (100%). Coronary angiography was performed in three patients – two cases of LCC PVCs and one with PVCs arising from the RCC-NCC commissure. In all patients, the coronary arteries were at a safe distance from the ablation catheter.
A. Premature ventricular contraction (PVC) activation map, projected in a posterior view, with the earliest activation point located in the right ventricular outflow tract (RVOT) slightly above the His bundle (5 mm away). This high-density activation mapping, performed with the PMF and the PentaRay mapping catheter, included 816 points (379 in the RVOT), resulting in a very detailed and accurate map. B. This high-density activation mapping included 308 points, with the earliest activation point just beneath the left coronary cusp. The figure is projected in the upper left posterior view. Also, in both maps performed with the PMF software and the PentaRay mapping catheter, the bipolar endocardial electrograms were less fractionated and had a higher voltage amplitude signal compared to the bipolar electrogram acquired with the ablation catheter, and so potentially more accurate. C. PVC activation map, projected in upper posterior view, with the earliest activation point in the aortomitral continuity. This activation map was performed with the standard approach and ST ablation catheter. Only 104 local activation time points were collected, since each point needs manual confirmation before validation and incorporation into the activation map, resulting in a less detailed map.
Premature ventricular contraction activation map, projected in an upper anterior view, with the earliest activation point located in the left coronary cusp. Both maps were performed in 12 minutes. A. This high-density activation map was produced with the PMF and the PentaRay catheter and included 547 points, resulting in a very detailed and accurate map. B. Standard activation map performed without PMF. Points were collected with the ablation catheter and required manual validation, resulting in fewer points being collected in the same amount of time – 42 points. Also, the bipolar endocardial electrogram had a lower voltage amplitude signal with larger electrogram duration compared to the bipolar electrogram acquired with the PentaRay catheter.
At 12 months post-procedure, there was no clinical recurrence, with all patients presenting a reduction in PVC burden of at least 80% compared with baseline, despite being off AAD. Of the three patients with LVEF<45%, two had complete normalization of LVEF.
Control Group (LAT with Ablation Catheter)Only four patients matched the inclusion criteria (Table 2) and were included in our study – all of them had PVCs from the LV (from the LCC, the RCC, the AMC (Figure 2C) and the posteromedial papillary muscle, respectively). Similar to patients from the PMF group, all four control patients had a high PVC burden (>23 000 PVC/24 h). Two patients had LVEF<45%.
Control group procedure data.
| No. | Age | Gender | PVC number | LVEF % | Site of origin | Points acquired | RF time, sec | Procedure time, min | CA | Acute success | Complications | PVC follow-up | LVEF %follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | Male | 21 000 | 35 | Aortic mitral continuity | 104 | 440 | 120 | Yes | Yes | None | 233 | 60 |
| 2 | 53 | Female | 25 600 | 50 | LCC | 14 | 180 | 70 | No | Yes | None | 75 | - |
| 3 | 50 | Female | 22 000 | 65 | RCC | 164 | 1584 | 200 | Yes | Yes | None | 24 000 | - |
| 4 | 80 | Male | 24 000 | 43 | Posteromedial papillary muscle | 76 | 620 | 130 | No | Yes | None | 1950 | 66 |
| Mean | 23 150±2055 | 48±13 | 90±62 | 706±613 | 130±54 |
CA: coronary angiography; LCC: left coronary cusp; LVEF: left ventricular ejection fraction; PVC: premature ventricular contraction; RCC: right coronary cusp; RF: radiofrequency.
During 36±24 min of mapping, the mean number of points acquired with the ablation catheter was 90±62. Median procedure and RF ablation times were 130±54 min and 706±613 s, respectively. Acute success was achieved in three of the four patients. The patient with PVCs arising from the RCC had a recurrence during the waiting period (mapping in this patient was also performed in the RVOT, but ablation was unsuccessful). Coronary angiography was performed in two patients – with LCC and RCC PVCs, respectively. In both cases, the coronary arteries were at a safe distance from the ablation catheter.
At 12 months post-procedure, the three patients who had had a successful ablation remained symptom free, with a PVC burden reduction of >80%, despite being off AAD. The two patients with a LVEF<45% had full LVEF recovery.
ComplicationsNo acute complications were observed in any of the groups.
DiscussionTo our knowledge, this is the first study assessing the use of the PMF software in combination with the PentaRay mapping catheter for complex PVC ablation. This prospective study illustrates the advantages of combining a multipolar electrode mapping catheter with the PMF software for complex PVC ablation, with four main benefits: (1) a much more detailed and accurate activation map; (2) a reliable map, since only PVCs with ≥97% correlation with the template morphology were collected and incorporated; (3) a reduction in the RF delivery time and finally, (4) a reduction in the procedure time.
The PentaRay is a tissue contact high-density multipolar electrode mapping catheter, capable of acquiring multiple LAT points (up to 20 simultaneously). It also covers a surface of more than 7 cm2.7 By acquiring simultaneous LAT points, it allows us to obtain a more detailed activation map and the mapping time is shorter. In our study, maps obtained in patients in the PMF group combined with the PentaRay catheter had a fivefold number of LAT points (507±213 vs. 90±62 points) and took less than half the time (15±10 vs. 36±24 min) compared with the control group. Also, unlike the ablation catheter (3.5 mm tip), the PentaRay has 1 mm electrodes with closer interelectrode spacing, which results in less signal averaging and fewer cancellation effects, and higher bipolar voltage amplitude with shorter electrogram duration. This subsequently allows more accurate time annotation.4 Moreover, the use of the PentaRay catheter can be particularly important in patients who present with few PVCs at the time of the procedure. In our clinical practice this is one of the most common reasons for procedure abandonment and re-scheduling. By allowing the simultaneous acquisition of multiple points, the activation map can be performed with fewer PVCs. Furthermore, due to the increased electrical density, the pace map will be more precise since ventricular capture can be achieved with a lower output, unlike what is observed when using a standard ablation catheter, particularly in the aortic root.3,4
As previously detailed, the PMF software follows five intrinsic steps before validation of the point: definition of the pattern, single channel correlation, calculation of overall weighted, repetition of the overall weight with phase shift of ±40 ms and finally the best result is compared to the threshold. In this study, by setting a higher correlation threshold with the PVC morphology to be mapped, only points with ≥97% correlation were automatically added to the activation map. This meant that each point of the activation map was highly reliable. This higher correlation threshold together with how the Wavefront is analyzed (bipolar and unipolar signals),5 mitigates one of the potential disadvantages of the PentaRay, the triggering of mechanical PVCs, and also avoids the acquisition of PVCs arising from close locations, which could decrease the accuracy of the map.
In our study, the multielectrode mapping catheter with the PMF software allowed a lower RF time (294±99 vs. 706±613 sec) and also practically halved the procedure duration (67±42 vs. 130±54 min), meaning there is potential for a higher patient turnover in the electrophysiology laboratory. Importantly, despite the reduction in the RF and procedure times, the safety and effectiveness of the procedure were not affected.
Further studies may help clarify whether this strategy is associated with higher success rates than standard approaches.
LimitationsWe acknowledge several limitations of our work. First, this was a single-center study performed by the same primary operator in a relatively high volume tertiary center, therefore our results may not be applicable to other centers. Second, few patients were included in both groups and therefore any comparison is inherently underpowered. However, our primary goal was to provide the first description of the combined use of the PentaRay catheter and the PMF software and illustrate its advantages. Moreover, our inclusion criteria were very restrictive, particularly in the control group, allowing only patients with complex PVCs who underwent ablation with the CARTO3 system with the same CF ablation catheter. Third, there is no uniformly accepted definition of successful ablation and so our results may differ from other studies using different definitions. Fourth, this approach cannot be performed in cases where the PVC arises from the distal coronary sinus. Fortunately, these cases are infrequent and even with the ablation catheter sometimes it is impossible to reach the target due to the coronary sinus anatomy. Finally, we acknowledge the cost of adding a multipolar mapping catheter on top of an ablation catheter with tridimensional mapping. This is why we only included complex cases – re-dos or left PVC ablations, which are normally associated with lower success rates.2 This does not preclude the use of this approach in patients with RVOT PVCs, particularly in those with few PVCs.
ConclusionIn this first study assessing the combined use of the PentaRay mapping catheter and the Pattern Matching Filter software for the ablation of complex PVCs, we demonstrate how this approach can improve the level of detail, accuracy and reliability of the activation map, while reducing the number of radiofrequency applications and procedural time. Further studies may be required to assess whether this approach can lead to improved outcomes.
Conflicts of interestP.A.S has received speaker fees from Biosense Webster, Boston Scientific and St. Jude Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.