A 78-year-old female with hypertension, atrial fibrillation (AF) and heart failure with preserved ejection fraction was admitted due to decompensated heart failure. On examination there was evidence of pulmonary congestion and there were no heart murmurs. The electrocardiogram showed AF rhythm with controlled ventricular response, with no other significant changes.
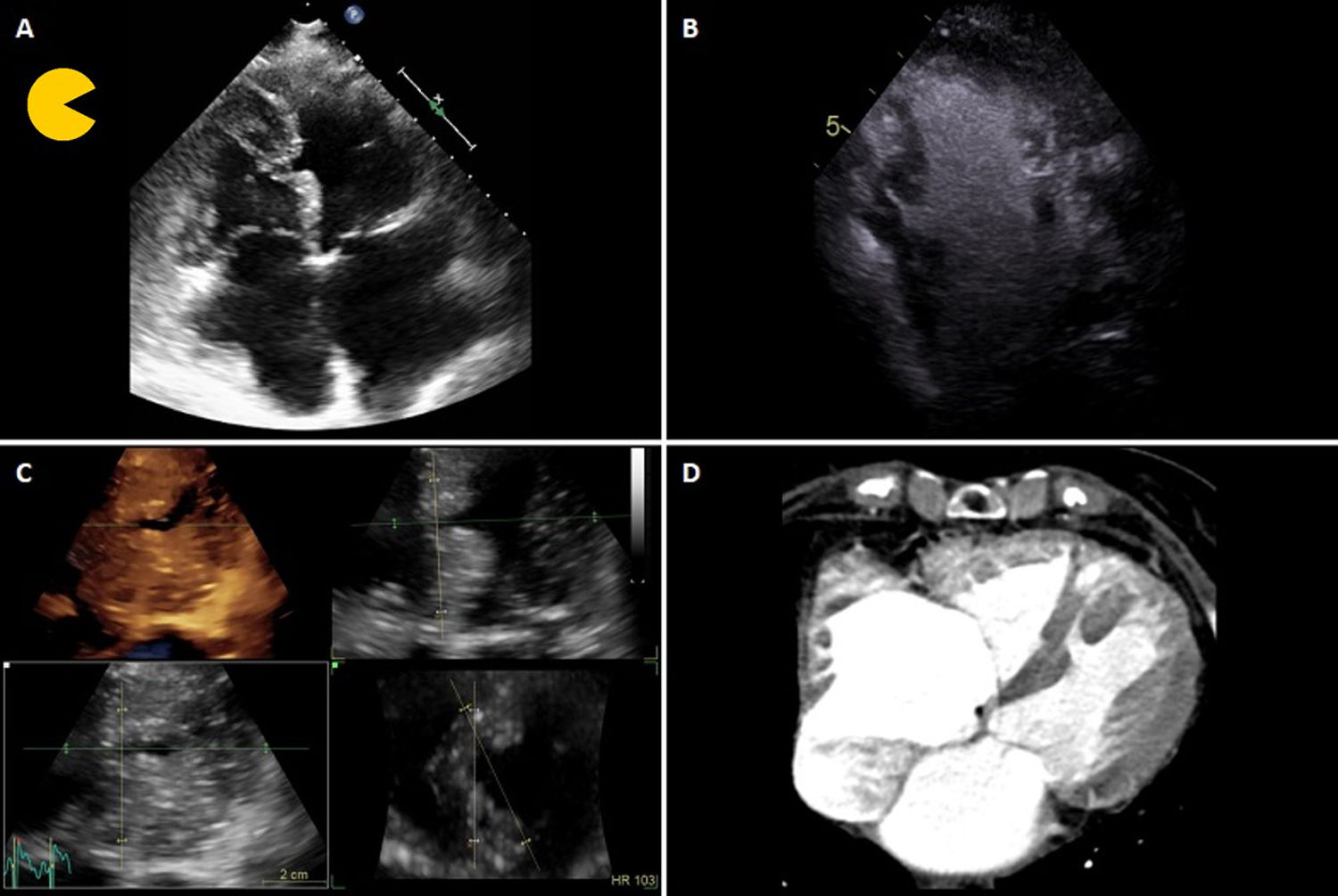
The transthoracic echocardiogram (TTE) revealed moderate biventricular hypertrophy with apical predominance and good systolic function. A partial loss of myocardial tissue in the mid segment of the interventricular septum was noticed, apparently without interventricular communication or left-to-right shunt on color Doppler (Figure 1A). Contrast-enhanced TTE showed a serpentine route through the septum to a small cavity contained within it (Figure 1B). Three-dimensional en face TTE views additionally clarified the half-moon shape of this defect and its movement during the cardiac cycle, closing during systole and opening during diastole (Figure 1C). Thoracic computed tomography conducted three years before in another context showed that the septal defect was already present, with similar characteristics (Figure 1D). Cardiac catheterization with oximetry and ventriculography was performed and interventricular shunt was excluded. There was no coronary artery disease. Cardiac magnetic resonance imaging was not possible due to lack of patient collaboration. The patient was discharged under optimized medical therapy.
(A) Transthoracic echocardiogram (TTE) showing a partial loss of myocardial tissue in the mid segment of the interventricular septum; (B) contrast-enhanced TTE revealing a serpentine route through the septum to a small contained cavity; (C) three-dimensional TTE en face view of the half-moon shaped septal defect; (D) thoracic computed tomography conducted three years before, showing the partial ventricular septal defect.
Partial ventricular septal defects, which are rarely reported, are thought to be congenital or a consequence of myocardial infarction. They have been termed ‘Pacman heart’ due to the shape changes during the cardiac cycle, becoming slit-like or absent during systole, like an opening and closing mouth, resembling the Pac-Man® video game. Related complications include conduction disturbances, rupture and altered systolic function.
Conflicts of interestThe authors have no conflicts of interest to declare.