Atrial fibrillation (AF), the most common arrhythmia in the adult population worldwide, represents a significant burden in terms of cardiovascular mortality and morbidity and has repercussions on health economics. Oral anticoagulation (OAC) is key to stroke prevention in AF and, in recent years, results from landmark clinical trials of non-vitamin K oral anticoagulants (NOAC) have triggered a paradigm shift in thrombocardiology. Despite these advances, there is still a significant residual vascular risk associated with silent AF, bleeding, premature sudden death and heart failure.
The authors review AF epidemiologic data, the importance of new tools for early AF detection, the current role of catheter ablation for rhythm control in AF, the state-of-the-art in periprocedural OAC, the optimal management of major bleeding, the causes of residual premature death and future strategies for improvements in AF prognosis.
A arritmia mais comum na população adulta em todo o mundo, a fibrilhação auricular (FA), contribui decisivamente para a elevada mortalidade e morbilidade cardiovascular, com repercussões na economia da saúde. A anticoagulação oral (ACO) é a chave para a prevenção do acidente vascular cerebral na FA. Nos últimos anos, os resultados dos grandes ensaios clínicos com os ACO não antagonista da vitamina K mudaram o paradigma na trombocardiologia. Apesar deste avanço, o risco vascular residual associado à FA silenciosa, hemorragia, morte súbita prematura e insuficiência cardíaca continua a ser significativo.
Os autores fazem uma revisão dos dados epidemiológicos da FA, a importância das novas ferramentas para a deteção precoce da FA, o papel atual da ablação por cateter no controlo do ritmo na FA, o estado da arte na ACO periprocedimento, a gestão ideal de hemorragias graves, as causas de morte prematura residual e estratégias futuras para a melhoria do prognóstico da FA.
Atrial fibrillation (AF) is the most common arrhythmia in the adult population worldwide, associated with high morbidity, increased mortality risk and impaired quality of life.1 Considering the aging population, the increase in predisposing factors for AF, and the improvement in healthcare with increased survival rates, AF is becoming a major and growing public health concern involving significant expenditure on health resources.2
Stroke is the leading cause of acquired disability and the second leading cause of mortality worldwide.3 In Portugal, stroke leads the causes of mortality representing 11% of all deaths, 71% of which are ischemic in nature.4,5 Although the global incidence of stroke is decreasing, cardioembolic stroke has tripled in recent decades with underlying AF in at least 20% of cases.6,7 The key preventive therapy for stroke associated with AF is anticoagulation.1
A decade after a paradigm shift in thrombocardiology, with the advent of non-vitamin K or direct oral anticoagulants (NOACs),8 the present document aims to reflect on future strategies for stroke prevention in AF (SPAF), and to further optimize overall AF prognosis in Portugal.
The authors of the present document are cardiologists, neurologists, internal medicine specialists, and immunohematologists, who work in the Portuguese National Health Service (NHS) and private hospitals (including emergency departments), and academic centers. The opinions expressed herein are based on their clinical and organizational experience and are supported by national and international evidence and guidelines. The authors undertake a review of AF epidemiologic data, consider the importance of new tools for early AF detection, the current role of catheter ablation (CA) for rhythm control in AF and the state-of-the-art periprocedural oral anticoagulation (OAC), optimal management of major bleeding, causes of residual premature death and future strategies for AF prognosis.
International and national atrial fibrillation epidemiologic dataThe 2010 Global Burden of Disease (GBD) study estimated a global age-adjusted prevalence of up to 33.5 million patients with AF in 2010, representing approximately 0.5% of the entire world population and reaching 2.5–3.5% of the population in many countries.2 Prevalence is likely to have been underestimated, as the GBD study did not include silent AF, a subclinical asymptomatic type of AF. This study showed that the age-adjusted incidence rates of AF were higher in developed countries compared with developing countries, with greater rates found in older individuals.2 Data from the Framingham Heart Study showed that the lifetime risk for development of AF in men and women aged ≥40 years is approximately one in four.9
In Europe, the estimated prevalence of AF is expected to increase from 8.8 million adults in 2010 to approximately 18 million by the year 2060,10 and the current estimated prevalence of AF in the general population is about 3.0%.
In Portugal, the FAMA study, a large-scale cross-sectional epidemiological study conducted in 2009, reported an AF prevalence of 2.5% in individuals aged ≥40 years, showing an increased prevalence with age, predominantly in the age group ≥70 years.11 The real prevalence of AF may have been underestimated as the frequency of paroxysmal AF was not accounted for in these data. In 2017, Primo et al. assessed the overall prevalence of AF and atrial flutter in individuals ≥40 years using 24-hour electrocardiographic monitoring; the prevalence observed was 12.4%.12 Similarly, a recent population-based study in elderly Portuguese subjects, the SAFIRA study, estimated an AF prevelance of 9.0%.13
AF has been associated with an increased risk of stroke, heart failure (HF), thromboembolism, cognitive decline, dementia, and death.1 Notably, AF increases the risk of ischemic stroke up to five-fold14,15 and overall, AF-related strokes are more severe and frequently fatal.16 However, the high efficacy of treatment with oral anticoagulation using vitamin K antagonists (VKA, e.g. warfarin) has made AF-related strokes largely preventable, reducing their relative risk by 64% and all-cause mortality by 26% compared with control or placebo.17 In recent years, NOACs have been developed to overcome some of the clinical limitations inherent to VKA therapy, especially the need for frequent laboratory monitoring, significant food and drug interactions and higher risk of intracranial bleeding.1 NOACs (dabigatran etexilate, rivaroxaban, apixaban, and edoxaban) have shown to be equally as or more effective than VKA for SPAF (supplementary material).8,18–20 The use of NOACs reduced stroke or systemic embolism by 19% and all-cause mortality by 10% in recent meta-analyses that compared the four NOACs with warfarin.21 Moreover, a low incidence of ischemic stroke and major bleeding was also found in a global registry of long-term treatment with dabigatran, further confirming the safety and effectiveness of NOACs for SPAF.22
A nationwide cohort study performed in Taiwan showed that, although overall anticoagulation use was suboptimal, a lower risk of ischemic stroke and mortality was associated with increasing prescription rates of OAC, thus supporting the introduction of NOACs into clinical practice.23 In Portugal, the increased use of NOACs for stroke prevention in AF is considered to be one of the major causes of the observed decline in ischemic stroke mortality, according to data from the Portuguese National Health Directory 2017.4
Screening and early diagnosis of atrial fibrillationIschemic strokes resulting from AF are common and frequently fatal, yet, at the same time, they are largely preventable with OAC therapy.1 However, a relevant proportion of stroke patients is only diagnosed with silent AF after having suffered a stroke, failing at the primary prevention of the vascular event.24 A recent meta-analysis found that approximately a quarter of patients are newly diagnosed with AF after sequential cardiac monitoring following a stroke or transient ischemic attack (TIA).25 Similarly, the Portuguese SAFIRA epidemiological study found that 36% of the population with AF was unaware of having this condition and that 17% of them were diagnosed with paroxysmal AF.13
The European Society of Cardiology (ESC) Guidelines for AF proposes the opportunistic screening of arrhythmia by pulse check or electrocardiogram (ECG) strip in people aged ≥65 years, as a class I recommendation.1 The same class of recommendation is advocated for the interrogation of cardiac implantable electronic devices and sub-cutaneous implantable cardiac monitors (ICMs) for detecting atrial high rate episodes.
The cause of approximately one third of all ischemic strokes remains unexplained after routine evaluation, therefore being classified, by exclusion, as cryptogenic. AF is very often associated with a stroke event initially labeled as cryptogenic.26,27 Two randomized trials have shown that a prolonged rhythm monitoring strategy may be crucial to identify an AF episode that would not have been detected with conventional follow-up after a cryptogenic stroke event (Supplementary material).28,29 The EMBRACE study found an AF incidence of 16% with a 30-day ECG monitoring strategy compared with an incidence of 3.2% in the control group undergoing 24-hr Holter monitoring.28 Similarly, the CRYSTAL-AF trial compared a six to 12 month monitoring strategy with a subcutaneous ICM versus the standard follow-up for detecting AF.29 AF was identified 9% and 12.4% of patients in the ICM group, versus 1.4% and 2.0% in the control group, after six and 12 months respectively.29 The AF episodes detected during the study were most frequently asymptomatic and paroxysmal (74% and 79% at six and 12 months within the ICM group).29 In patients with ischemic stroke or TIA, the ESC Guidelines suggest screening for AF using continuous ECG monitoring for at least 72 hours as a class I recommendation.1 More prolonged ECG monitoring with noninvasive monitors or implantable loop recorders should be considered in these patients to detect silent AF (class IIa recommendation).1
We are currently witnessing a paradigm shift in screening tools to improve the early detection of subclinical AF.30,31 Numerous devices have been developed based on different technologies. Handheld single-ECG devices (e.g. AliveCor Kardia, Mydiagnostick) operating with automated algorithms have demonstrated a good predictive diagnostic accuracy (positive predictive value ranging from 54.8 to 88.9% and negative predictive value between 91.1 and 96.1%).32 Automated blood pressure monitors (e.g. Omron M6, Microlife BP A200 Plus) can identify pulse irregularity associated with AF (positive predictive value 81.5 to 83% and negative predictive value 98 to 100%).33 Patch ECG monitors (e.g. Zio – iRhythm, Cardiostat – Icentia, Nuvant - Corventis) allow prolonged rhythm monitoring and were more sensitive than 24-h Holter monitoring for AF detection.34 Finally, photoplethysmography (PPG) technology applied to mobile phones and smartwatches may play a key role in more widespread detection of AF. The Apple Heart Study, which assessed the performance of a smartwatch PPG-based algorithm in a broad population of over 400 000 participants, for the detection of pulse irregularity that might reveal previously unknown AF, presented a positive predictive value of 71% (Supplementary material).35 Notably, an important finding to overcome concerns about potential over-notification is that only 0.5% of the participants received an irregular pulse notification. The Huawei Heart Study also demonstrated the usefulness of PPG-based technology in AF screening, with a positive predictive value of 91.6% (Supplementary material).36 Any attempt to make indirect comparisons on the predictive performance of the various devices should take into account the dependence of the predictive value of a test on the prevalence of the disease.37
Catheter ablation in atrial fibrillationCA is a well-established and effective treatment strategy for symptomatic AF patients.1 In fact, it is more effective than antiarrhythmic drug therapy in patients with symptomatic paroxysmal, persistent, and probably long-standing persistent AF.38
The greater efficacy of CA in maintaining sinus rhythm has given rise to the hypothesis that this therapy might be superior to a rate control strategy in reducing major clinical outcomes, which in patients under appropriate OAC is similar to pharmacologic rhythm control.39 In the CABANA trial, which enrolled 2204 patients with new-onset or untreated AF requiring therapy, CA was not superior to pharmacologic therapy in reducing the combined primary outcome of death, disabling stroke, serious bleeding or cardiac arrest (HR 0.86, CI 95% 0.65 to 1.15) at four years (Supplementary material).40 However, CA significantly reduced the relative risks of the secondary endpoints of death or cardiovascular hospitalization by 17% and recurrent AF by 48%. Moreover, CA was superior to drug therapy in reducing the relative risks of the primary endpoint by 33% and all-cause death by 40% in the treatment received analysis. However, several methodological issues, such as elevated cross-over rates between treatment arms, have limited the generalizability of the CABANA trial results. On the other hand, the CASTLE-AF trial,41 which randomized 363 patients with AF and HF with reduced ejection fraction (HFrEF) to CA or standard treatment, showed evidence of prognostic benefit after three years of follow-up, with CA significantly reducing the primary outcome of death or hospitalization for HF (HR 0.62, CI 95% 0.43 to 0.87) (Supplementary material). Results from the ongoing EAST-AFNET 4 Trial may help to establish the role of CA in improving outcomes, if applied early after the initial diagnosis of AF.42
Although CA is considered a relatively safe procedure, there are some rare but severe periprocedural complications associated with the technique, with the most serious adverse events being stroke and severe bleeding.43 To reduce the risk of thromboembolic complications, patients should not discontinue OAC therapy (uninterrupted strategy) before CA.1 In the COMPARE trial, periprocedural stroke and bleeding complications were significantly reduced by the uninterrupted warfarin strategy compared to bridging with low-molecular-weight heparin (Supplementary material).44
The uninterrupted anticoagulation strategy for CA was also assessed with NOACs (Table 1).45–48 The VENTURE-AF trial was the first prospective randomized trial to compare the use of uninterrupted rivaroxaban versus warfarin in patients undergoing AF CA.45 Results from this trial led to the conclusion that the use of uninterrupted rivaroxaban was feasible and that the bleeding event rates and ischemic outcomes were low and similar to those of uninterrupted warfarin therapy. Similar results were observed with other factor Xa (FXa) inhibitors, apixaban in the AXAFA-AFNET 5 trial, and edoxaban in the ELIMINATE-AF trial, when compared with uninterrupted warfarin (Table 1). In the RE-CIRCUIT trial, uninterrupted dabigatran was associated with fewer bleeding complications than uninterrupted warfarin, corresponding to a significant absolute risk reduction of 5.3% and a relative risk reduction (RRR) of 77% in major bleeding.46
Randomized trials comparing uninterrupted strategies of non-vitamin K oral anticoagulant versus warfarin in patients undergoing catheter ablation for atrial fibrillation.
| Study | AXAFA – AFNET 547 | RE-CIRCUIT46 | ELIMINATE-AF48 | VENTURE-AF45 |
|---|---|---|---|---|
| Uninterrupted NOAC | Apixaban | Dabigatran | Edoxaban | Rivaroxaban |
| Population | 633 | 635 | 632 | 248 |
| Mean age (years) | 64** | 59.2 | 59.5 | 59.6 |
| CHA2DS2-VASc | 2.4 | 2.1 | NR | 1.6 |
| Ischemic stroke or TIA* | 0.6% vs. 0% | 0% vs. 0.3% | 0.3% vs. 0% | 0% vs. 0.8% (NS) |
| Major Bleeding* (HR; CI 95% CI) | 3.1% vs. 4.4% (NS) | 1.6% vs. 6.9% (0.22; 0.08-0.59) | 2.5% vs. 1.5% (NS) | 0% vs. 0.4% (NS) |
| Total unit of heparin units* | NR | 12 402 vs. 11910 (NS) | 14 261 vs. 11 473 (p<0.0001) | 13 871 vs. 10 964 (p<0.001) |
| Median TTR in warfarin group | 84% | 66% | 65% | NR |
In the RE-CIRCUIT trial, patients receiving dabigatran required a similar amount of heparin as those under warfarin to achieve the target activated clotting time (ACT) during ablation, whereas patients treated with FXa inhibitors required approximately 25% more heparin than those allocated to warfarin.45,48,49 This difference may be attributed to the distinct pharmacodynamic effects on the detection or non-detection of the anticoagulant effect by ACT, for the thrombin inhibitor dabigatran and for the FXa inhibitors, respectively.49
Although the previous results are relevant to clinical practice, they are derived from clinical trials with highly selected young populations.50 Considering this, Yanagisawa et al. conducted a retrospective study to specifically assess the efficacy and safety of uninterrupted NOAC use in elderly patients undergoing CA in AF.51 In this study, the elderly group (age ≥75 years) had a significantly higher number of periprocedural bleeding events when compared to the younger group, but no statistically significant differences were found between the patients taking NOAC or warfarin in both subgroups. Even if these results are not completely unexpected, they underscore age as an important risk factor for bleeding and the need to monitor these patients more closely during the periprocedural period.
Overall, findings from the previous clinical trials support the idea of using uninterrupted NOAC during CA in AF, which corroborates the latest 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of AF.52
Oral anticoagulation optimization to fit patient profileKnowledge of patient history and clinical characteristics is essential to optimize OAC therapy and reduce AF-related-risks, complications and mortality.53
Several stroke risk stratification scores in AF patients have been developed to estimate the risk of thromboembolism to support the decision to initiate OAC.54–56 The CHA2DS2-VASc score is one of the most referenced stroke risk stratification scores in international guidelines concerning antithrombotic prophylaxis in AF.55,56 This score integrates known stroke risk factors, such as age (≥75 and 65 to 74 years), congestive HF (signs/symptoms of HF or reduced left ventricular ejection fraction), hypertension (blood pressure >140/90 mmHg on at least two occasions or current antihypertensive treatment), diabetes (fasting glucose ≥126 mg/dL or treatment with oral hypoglycemic agent and/or insulin), prior stroke, TIA or thromboembolism, vascular disease (previous myocardial infarction, peripheral arterial disease or aortic plaque) and female individuals.55
Other risk markers, such as impaired renal function, certain biomarkers and left atrial enlargement can improve risk stratification but the gain in predictive value does not compensate the complexity of the scores that include them.57–59 However, they can be useful in specific patients for risk stratification.
The benefit of OAC in patients presenting a CHA2DS2-VASc risk score ≥2 in men and ≥3 in women, is strongly supported by clinical evidence.1,60 On the other hand, in intermediate-risk patients (CHA2DS2-VASc score 1 in men and 2 in women) the evidence is not as robust and the therapeutic strategies still pose challenges in clinical practice, with the need to weigh up the individual benefit of reducing thromboembolic risk and the risk of bleeding – the concept of net clinical benefit.1,60–62 Based on data from the large phase III trials of NOACs, the ESC Working Group on Cardiovascular Pharmacotherapy and the ESC Council on Stroke state that in patients with a single stroke risk factor, NOAC with a superior net-clinical benefit should be preferred over vitamin K antagonists (VKAs).61
Concurrently, patient bleeding risk should also be assessed when defining the therapeutic strategy and as part of clinical practice management.56 The HAS-BLED score is the most commonly used in AF patients, and has been validated in patients on aspirin, OAC (VKA or NOAC) and no antithrombotic therapy, with a high predictive value for hemorrhagic events, especially intracranial hemorrhage, in different situations.56,63 Since it also relies upon modifiable bleeding risk factors (uncontrolled hypertension, excessive alcohol consumption or concomitant use of other drugs that may influence the bleeding risk), and the stroke risk usually outweighs the high bleeding risk, a high HAS-BLED score of ≥3 generally is not a reason to avoid or discontinue anticoagulation; instead, it indicates that the patient should have regular reviews of potential causes of bleeding and efforts to reduce the modifiable bleeding risk factors should be made.1,53,56
Another factor that must be considered for treatment optimization is the patient's ability to comply with treatment. Success in VKA treatment depends on anticoagulation quality control; on the other hand, patients with poor compliance will not benefit from NOACS. They have a short half-life, therefore missing a few doses will ultimately lead to sub-therapeutic drug concentrations.64 In both situations, if proper treatment adherence is not achieved, a higher risk of stroke and mortality is observed.65–67
There are other important factors in OAC choice that highlight the need to assess whether dose reductions or switch are required, such as advanced age, abnormally low weight, renal insufficiency, specific bleeding risk (e.g. gastrointestinal) and drug interactions.53,64,68,69 The absence of these risks should also be considered, as in the case of patients under the age of 75 years, in which dabigatran 150 mg significantly reduced the relative risks of stroke/systemic embolism (SE), major bleeding and all-cause death by 37%, 30% and 23% versus warfarin, respectively, or patients with normal renal function (CrCl>80 ml/min) in which the risk of stroke/SE was higher in patients on edoxaban compared with warfarin.70,71
The existing evidence from clinical trials and large observational studies in different settings and populations, of different available therapeutic options, allows for the choice and management of the best OAC to fit patients’ characteristics (Table 2).
Choosing a specific oral anticoagulant and dose for stroke prevention in atrial fibrillation in patient subsets.
| Patient subset | First choice |
|---|---|
| Nonvalvular AF, paroxysmal, persistent or permanent, with a CHA2DS2-VASc risk score ≥2 in men and ≥3 in women | OAC is recommended and NOACs are preferred over VKAs |
| Nonvalvular AF on VKA with TTR >70% | Continue with VKA; consider NOAC if complication; SAMe-TT2R2 score >2; patients preference |
| CHA2DS2-VASc 1 in men and 2 in women | OAC should be consideredDabigatran (150 mg twice daily is preferred) or apixaban may be considered |
| Stable coronary artery disease or peripheral artery disease | Monotherapy with a NOAC |
| Mechanical prosthetic heart valves or moderate/severe (rheumatic) mitral stenosis | VKANOACs should not be used |
| Secondary stroke prevention | NOACs as a group are superior to warfarin |
| High risk of gastrointestinal bleeding | Apixaban 5 mg twice daily or dabigatran 110 mg twice daily may be used |
| Renal impairment | CrCl 30–49 mL/min: Apixaban 5 mg twice daily (apixaban 2.5 mg twice a day if ≥1 additional criteria: age ≥80 years, body weight ≤60 kg, serum creatinine ≥1.5 mg/dL are present), rivaroxaban 15 mg daily, edoxaban 30 mg once daily or dabigatran 110 mg twice daily |
| Elderly | ≥75 years, we suggest apixaban 5 mg twice daily [2.5 mg if ≥2 of the following: age ≥80 years, body weight ≤60 kg, or creatinine ≥1.5 mg/dL] |
| Age <75 years with preserved renal function | Dabigatran 150 mg twice daily may be considered |
AF: atrial fibrillation; CrCl: creatinine clearance; NOAC: non-vitamin K oral anticoagulant; OAC: oral anticoagulant; VKA: Vitamin K antagonist.
Anticoagulant are associated with increased risk of bleeding, generally induced by traumatic, inflammatory or neoplastic vascular injury. In the GARFIELD–AF study, enrolling 28 628 patients with AF and 63% under OAC, the rate of first occurrence of clinically relevant bleeding in patients with or without OAC was 3.0%, with a fatal outcome in 6.9% of the events over two year follow-up.72 Subjects on OAC were 73% more likely to experience clinically relevant bleeding. Indeed, bleeding was associated with higher mortality, representing 6% of the causes of death in patients with AF on OAC,73 and was even more problematic in the presence of multiple morbidities, high risk medications, polypharmacy, or drug-drug interactions.74 Notwithstanding these factors, the net clinical benefit of contemporary OAC versus no treatment was overwhelming, avoiding 50 strokes and 30 deaths, at the cost of two intracranial and one fatal bleeding event per 1000 patients treated over one year.75
In clinical trials, NOACs reduced the relative risks of bleeding by 14% for major, 52% for intracranial and 47% for fatal events compared with warfarin.21,76 Despite their increased safety, the use of NOACs is generally associated with increased risk of gastrointestinal bleeding21 and a fatal outcome is present in 9 to 20% of patients who suffer a major bleeding event.77–79 In this setting, adherence to recommended management of bleeding guidelines is mandatory (Table 3).1,64
Management of active bleeding in patients on oral anticoagulation.9,73
| White•Identify the bleeding site and apply local hemostatic measures |
| •Obtain history of OAC (type and last dose) – delay next OAC dose |
| •Assess hemodynamic (blood pressure) and laboratorial (basic coagulation, blood count and kidney function parameters) status |
| Light gray•Add symptomatic treatment – fluid replacement and blood transfusion |
| •Treat bleeding cause (e.g. endoscopy or surgery) |
| Gray•Consider specific reversal agent (e.g. idarucizumab for dabigatran and andexanet alfa for FXa inhibitors) |
| •Consider PCC if no specific reversal agent available |
OAC: oral anticoagulant; PCC: prothrombin complex concentrates.
White: Minor/Moderate/Severe; Light gray: Moderate/Severe; Gray: Severe.
The 2016 ESC Guidelines for AF state that the first step in the management of patients with active bleeding is the application of local hemostatic measures by means of mechanical compression.1 However, this is not sufficient or even possible in several bleeding scenarios. In hemorrhages occurring in deep organs, this therapeutic approach is only possible using invasive endoscopic or surgical methods, which is not feasible in the context of systemic anticoagulation.
Also, in hemorrhages at critical sites or associated with hemodynamic instability, reversal of OAC is mandatory, to improve hemostasis. For this purpose, reversal of OAC is also critical, to allow spontaneous hemostasis.64
The reversal of VKAs includes the administration of vitamin K, prothrombin complex concentrates (PCC) or fresh frozen plasma (if PCC unavailable).80 Vitamin K administration is intravenous, leading to a sustained but not immediate correction of coagulopathy. For patients with major bleeding, in order to achieve a rapid correction, PCC should be concomitantly administrated, according to international normalized ratio (INR) and weight of the patient (PCC – 25 U/kg for INR 2-4; 35 U/kg for INR 4-6 and 50 U/kg if INR>6).76 When PCC is not available, fresh frozen plasma is used (10-15 mL/kg); however, it presents several disadvantages (e.g. it requires ABO blood typing and thawing, it has lower concentration of coagulation factors and higher volumes are needed when compared to PCC, which can cause circulatory overload).80
Idarucizumab is approved as the specific reversal agent for dabigatran. It is an antigen-binding fragment of a humanized monoclonal antibody with a binding affinity approximately 350-fold more potent than dabigatran's affinity for thrombin.1,64,81 In the RE-VERSE AD trial, an open label and single-arm study that enrolled 503 patients with uncontrolled bleeding or undergoing an urgent procedure, idarucizumab (2×2.5 g intravenous) rapidly, durably and safely reversed the anticoagulant effect of dabigatran (Supplementary material).81 If unavailable, PCC (25-50 U/kg) can be used as an alternative.80
Andexanet alfa is a modified recombinant inactive form of human factor Xa that binds FXa inhibitors with an affinity similar to that of native FXa. It was recently approved as a specific reversal agent for the FXa inhibitors apixaban and rivaroxaban. The ANNEXA-Atrial, an open label and single-arm study, enrolled 352 patients with acute major bleeding (Supplementary material).82 This trial demonstrated that andexanet alfa reduced anti-FXa activity substantially. This agent is administered as a bolus (400 mg for apixaban or rivaroxaban >7 h; 800 mg for enoxaparin, edoxaban or rivaroxaban <7 h) followed by a two-hour infusion (480 mg for apixaban or rivaroxaban >7 h; 960 mg for enoxaparin, edoxaban or rivaroxaban <7 h). For FXa inhibitors under-evaluated in the ANNEXA-4, namely edoxaban, a coagulation factor supplementation with PCCs is recommended, as well as for the other agents in the absence of andexanet alfa.80 The reversal agent ciraparantag, reported to bind all the NOACs, is undergoing phase III assessment.80
Reversal of the anticoagulation effect with specific agents may improve survival in patients with life-threatening bleeds. Indeed, although the RE-VERSE AD and ANNEXA-4 trials did not include a control group, 30-day mortality for intracranial hemorrhage (ICH) was 16.4% in patients managed with idarucizumab and 21.6% in those managed with andexanet alfa.81,82 These ICH mortality rates are notably lower than those observed in the pivotal SPAF NOAC trials (from 35 to 45%), independent of OAC with VKAs or NOACs, where patients were managed without the use of specific reversal agents.78,81–83
European Guidelines recommend restarting OAC after a bleeding event in all eligible patients following assessment by a multidisciplinary AF team, considering different OAC and stroke prevention interventions, improved management of factors that contributed to the bleeding, and stroke risk.1 It is essential to treat the culprit vascular lesion before restarting OAC therapy. The majority of culprit lesions can be rapidly identified during diagnostic work-up of gastrointestinal and urinary tract bleeds.84,85 The role of a multidisciplinary team, comprised of stroke physicians/neurologists, cardiologists, internal medicine specialists, surgeons, neuroradiologists, immunohematologists and nurses is essential to this process, where not only when starting but also when re-starting OAC, the communication strategies, sharing of knowledge, trust, and mutual respect are crucial to patient education, compliance and treatment adherence.1,86
In patients with a contraindication for OAC treatment, left atrial appendage (LAA) occlusion may be considered for SPAF.1 Most of the evidence on the benefit of LAA occlusion in SPAF comes from the PROTECT-AF and PREVAIL trials (Supplementary material).87,88 In these studies LAA occlusion was non-inferior to VKA treatment for the prevention of stroke with lower bleeding rates.1
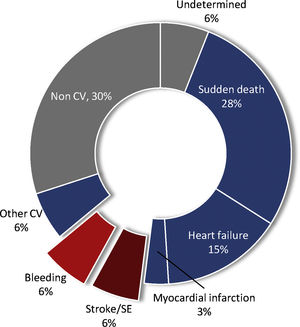
Further prognostic optimization in atrial fibrillationDespite the great progress made in stroke prevention, death remains the most frequent major event in patients with AF on OAC.21 Cardiovascular causes account for 64% and vascular causes (embolism and bleeding) for 12% of all-cause deaths (Figure 1).73 Sudden cardiac death (SCD) and HF account for 43% of total mortality. NOACs showed a significant 10% reduction in all-cause death when compared to warfarin, mainly driven by a significant reduction of 50% in the relative risk of fatal bleeding.73 In the RE-LY trial, dabigatran was associated with a significant relative reduction of 37% in vascular death.89 Optimal treatment for HF defined by the combined use of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) and beta-blocker (BB) was associated with a significant relative reduction of 41% in SCD in patients with HF.89
Causes of death in patients with atrial fibrillation under oral anticoagulation.73
CV: cardiovascular; SE: systemic embolism.
Another area of particular interest in AF is HF; they frequently coexist and deteriorate each other.90–92 The prevalence of AF increases with the severity of HF and is a marker of disease progression. On the other hand, AF is a predictor of mortality in patients with HF and HF is a major risk factor for stroke in AF.93,94 In this setting, CA significantly reduced the relative risks of all-cause death and hospitalizations for HF by 47% and 44%, respectively in the aforementioned CASTLE-AF study.41
Ablation was associated with a lower risk of ischemic stroke compared with medical therapy in a real-world population enrolled in a Swedish registry.95 The AF burden would benefit from primary prevention and management of the shared underlying risk factors with SCD and HF. Further interventions, beyond appropriate anticoagulation, are necessary to reduce the risk of outcomes other than stroke, in patients with AF. This improvement includes the appropriate management and treatment of relevant comorbidities such as diabetes, hypertension, obesity, obstructive sleep apnea and thyroid disease, as well as healthy lifestyle changes96; abstinence from alcohol significantly reduced AF recurrence.97 Retrospective analysis from randomized trials of sodium-glucose cotransporter 2 inhibitors have reported a lower incidence of new-onset AF in patients with type 2 diabetes (T2DM)98 and significant reductions in CV death or hospitalization for HF in patients with AF, CV disease and T2DM.99 Similarly, the use of ACEI/ARB, BB or eplerenone in patients with HFrEF has been associated with a lower incidence of new-onset AF.1
Conclusions & future strategiesEpidemiological data at a global, European and national level reveal the pandemic nature of AF and some recent studies point to a reduction in stroke events with greater access to OAC via NOACs.
Considering that a significant proportion of the total AF population is asymptomatic or mildly symptomatic, early detection in these undiagnosed patients is crucial for a timely start of OAC therapy in order to avoid ischemic stroke from being the first clinical manifestation of AF, as well as strategies to prevent AF progression to HF and other complications. Therefore, new technologies and tools aiming at screening and for simple, timely, and accurate AF detection will need to be implemented in routine clinical practice.
There is increasing evidence that AF CA has a prognostic impact, particularly in some patients with HF and severely reduced ejection fraction. However, to achieve the best risk-benefit ratio, the procedure should be performed under uninterrupted anticoagulation.
Even though antithrombotic prophylaxis with OAC reduces the risk of ischemic stroke in AF, major bleeding is the most frequent adverse reaction, representing 6% of the causes of death in patients with AF on OAC. Specific patient clinical characteristics may influence the bleeding risk. Therefore, choosing the appropriate OAC and the right dose that fits the patient profile better is essential in order to optimize therapy and reduce the risk of non-fatal and fatal hemorrhages in AF patients.
Managing major bleeding in patients on OAC can pose challenges. Current management focuses on the expedited search for the underlying vascular injury and local hemostasis. However, in the setting of invasive procedures, reversal of OAC is critical to undertake this therapeutic approach.
NOACs reduce premature death in patients with AF, but in the ideal scenario of widespread use of these agents, more than 40% of fatalities are due to SCD and HF. Therefore, patients with AF need evidence-based therapeutic strategies for the prevention of HF and SCD, on the development of pragmatic randomized controlled trials with these events as primary outcomes.
Conflicts of interestJF – Consulting and lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme and Novartis.
NA - No competing financial interests or personal relationships that could influence the work reported in this paper.
NCD - Consulting and lecture fees from Abbott, Medtronic, Biosense Webster, Boston Scientific, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb and Daiichi-Sankyo.
LRG - Consulting and lecture fees from Bayer, Boehringer Ingelheim, Bristol-Meyer Squibb, CSL-Behring, Daiichi-Sankyo, Leo, Pfizer
JSF - Travel and lecture fees from Boehringer Ingelheim, Bayer, BMS-Pfizer and Daiichi-Sankyo.
PvH - Consulting and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Menarini and Novartis.
VG - Consulting or lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, BMS, Pfizer, Daichii-Sankyo
FundingThis paper had a non-restrictive financial support of Boehringer Ingelheim.
The Authors acknowledge writing assistance provided by Ana Santos from Prime Focus, funded by Boehringer Ingelheim. The support provided by Rachel Melo from Boehringer Ingelheim deserves special recognition.