Biomarkers have a variety of clinical applications in multiple stages of diagnosis and therapy. Troponin T and brain natriuretic peptide are the best-known in the cardiovascular field, but experimental studies have identified new biomarkers with potential clinical value. In this article, novel biomarkers of kidney injury are investigated in the context of their relationship with atherosclerotic coronary disease. This review was carried out through a search in the PubMed database using as keywords each biomarker to be studied with the descriptor (DECS/MeSH) “Myocardial Infarction”, and the keywords “coronary” and “cardiovascular”, using the Boolean operator “AND”. After the selection, 24 articles published between 2003 and 2017 were identified for the review. Eight biomarkers were investigated: neutrophil gelatinase-associated lipocalin (NGAL), fibroblast growth factor 23 (FGF23), tissue inhibitor of metalloproteinase-2 (TIMP-2), syndecan-1, interleukin-6 (IL-6), galectin-3, and the vascular cell adhesion molecules ICAM-1 and VCAM-1. Most identified articles were experimental studies, studies on human subjects having few participants. There are several promising biomarkers in the setting of coronary disease. The main evidence available in the literature suggests that elevated NGAL levels are associated with better prognosis after cardiac arrest and with comorbid kidney injury; elevated FGF23 is associated with coronary artery disease severity; TIMP-2 protects against coronary artery disease; increased expression of syndecan-1 is observed in myocardial infarction (MI) and protects against an exacerbated inflammatory response; IL-6 is associated with atherosclerotic disease and major cardiovascular outcomes; galectin-3 correlates with adverse clinical events post-MI; and elevated ICAM-1/VCAM-1 levels are associated with risk of coronary disease. Further studies are required to better investigate the role of each of these biomarkers in both stable coronary disease and acute coronary syndrome.
Os biomarcadores têm uma variedade de aplicações clínicas em vários estágios do diagnóstico e terapêutica. A troponina T e o peptídeo natriurético cerebral são os mais conhecidos no campo cardiovascular, mas estudos experimentais mostraram novos biomarcadores com potencial de uso clínico. Em nosso artigo, os novos biomarcadores de lesão renal foram investigados no contexto de sua relação com doença coronariana aterosclerótica. Esta revisão foi realizada por meio de uma pesquisa no Pubmed, utilizando como palavras-chave cada biomarcador a ser estudado com o descritor (DECS/MESH): “Myocardial Infarction” e as palavras-chave “coronary” e “cardiovascular”, utilizando o Booleano operador “AND”. Após a seleção, 24 artigos publicados de 2003 a 2017 foram identificados para a revisão. Oito biomarcadores foram investigados: lipoproteína associada a gelatinase de neutrófilos, fator de crescimento de fibroblastos 23 (FGF 23), inibidor tecidual de metaloproteinases 2 (TIMP-2), sindecano 1, interleucina 6 (IL-6), galectina 3 e moléculas de adesão vascular – ICAM-1 e VCAM-1. Os artigos mais verificados foram estudos experimentais e com estudos em humanos com poucos participantes. Existem vários biomarcadores promissores no cenário da doença coronariana. As principais evidências disponíveis na literatura sugerem que níveis elevados de NGAL estão associados a um melhor prognóstico após morte súbita cardíaca e lesão renal associada; níveis elevados de FGF 23 mostraram estar associados à gravidade da doença arterial coronariana; o gene TIMP-2 protege contra doença arterial coronariana; é observada uma expressão aumentada de sindecano-1 no infarto agudo do miocárdio (IAM) e protege contra uma resposta inflamatória exacerbada; a IL-6 está associada ao aumento da doença aterosclerótica e aos principais desfechos cardiovasculares; a galectina-3 se correlaciona com eventos clínicos adversos pós-IAM; e níveis elevados de ICAM/VCAM-1 estão associados ao risco de doença coronariana. Mais estudos são necessários para investigar melhor o papel de cada um desses biomarcadores na doença coronária estável e na síndrome coronariana aguda.
In 1992, the US Food and Drug Administration (FDA) defined a surrogate endpoint as a laboratory measure or a physical sign that is intended to be used as a substitute for a clinically meaningful parameter that measures directly how the patient feels, functions or survives and is expected to predict the effect of therapy. As early as 1998, the US National Institutes of Health convened a working group that developed new definitions in the area of biological markers. They defined a biological marker – abbreviated to ‘biomarker’ – as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”1
The clinical applications of biomarkers in the cardiovascular system encompass diverse stages of diagnosis and therapy. They can be applied to disease diagnosis or risk stratification, as therapeutic targets, to assess the efficacy of novel therapies, and for evaluation and registration of drugs.1
Some biomarkers are well established and commonly used in clinical practice, including troponin I and T, which are used in the diagnosis of myocardial infarction (MI), and creatine kinase-myocardial band (CK-MB), which is not sufficiently sensitive to diagnose MI in the first hours, but is useful in the diagnosis of reinfarction and post-procedural MI. Other biomarkers that are easily accessible in practice, such as brain natriuretic peptide and its inactive N-terminal fragment, are used to predict ventricular dysfunction after MI and adverse events in both stable angina and acute coronary syndrome (ACS).2
However, in recent years, numerous studies have sought to increase the pool of biomarkers available for use in patients with coronary artery disease (CAD) by assessing biomarkers used in other clinical contexts, such as in kidney disease.
ACS is more prevalent in patients with acute kidney injury (AKI), chronic kidney disease (CKD) and end-stage renal disease. When associated with kidney disease, CAD has worse outcomes and is often harder to manage. However, in clinical practice, each disease has specific biomarkers for diagnosis and prognosis, creating difficulty in clinical correlation of biomarkers. In view of the relationship between atherosclerotic disease and kidney disease, the hypothesis was raised that there would be biomarkers related to kidney disease that could predict events and prognosis in ischemic heart disease, since they share a similar pathophysiology: the inflammatory process.
The aim of the present study was to identify potential new biomarkers commonly studied for kidney disease that have also been studied as prognostic predictors in patients with atherosclerotic heart disease, in both the acute and chronic phase.
MethodsThis review was carried out according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines, following the steps proposed by Pereira and Galvao, namely: (1) creation of the research question; (2) literature search; (3) article selection; (4) data extraction; (5) methodological quality assessment; and (6) data synthesis.
In order to develop the research question, the PICo approach was used, where P=population or problem, I=interest and Co=context. This study sought to investigate new biomarkers in the context of atherosclerotic coronary disease, where P=world population, I=biomarkers and Co=coronary disease.
Eight biomarkers – neutrophil gelatinase-associated lipocalin (NGAL), fibroblast growth factor 23 (FGF23), tissue inhibitor of metalloproteinase-2 (TIMP-2), syndecan-1, interleukin-6 (IL-6), galectin-3, and the vascular cell adhesion molecules ICAM-1 and VCAM-1 – were selected for the study.
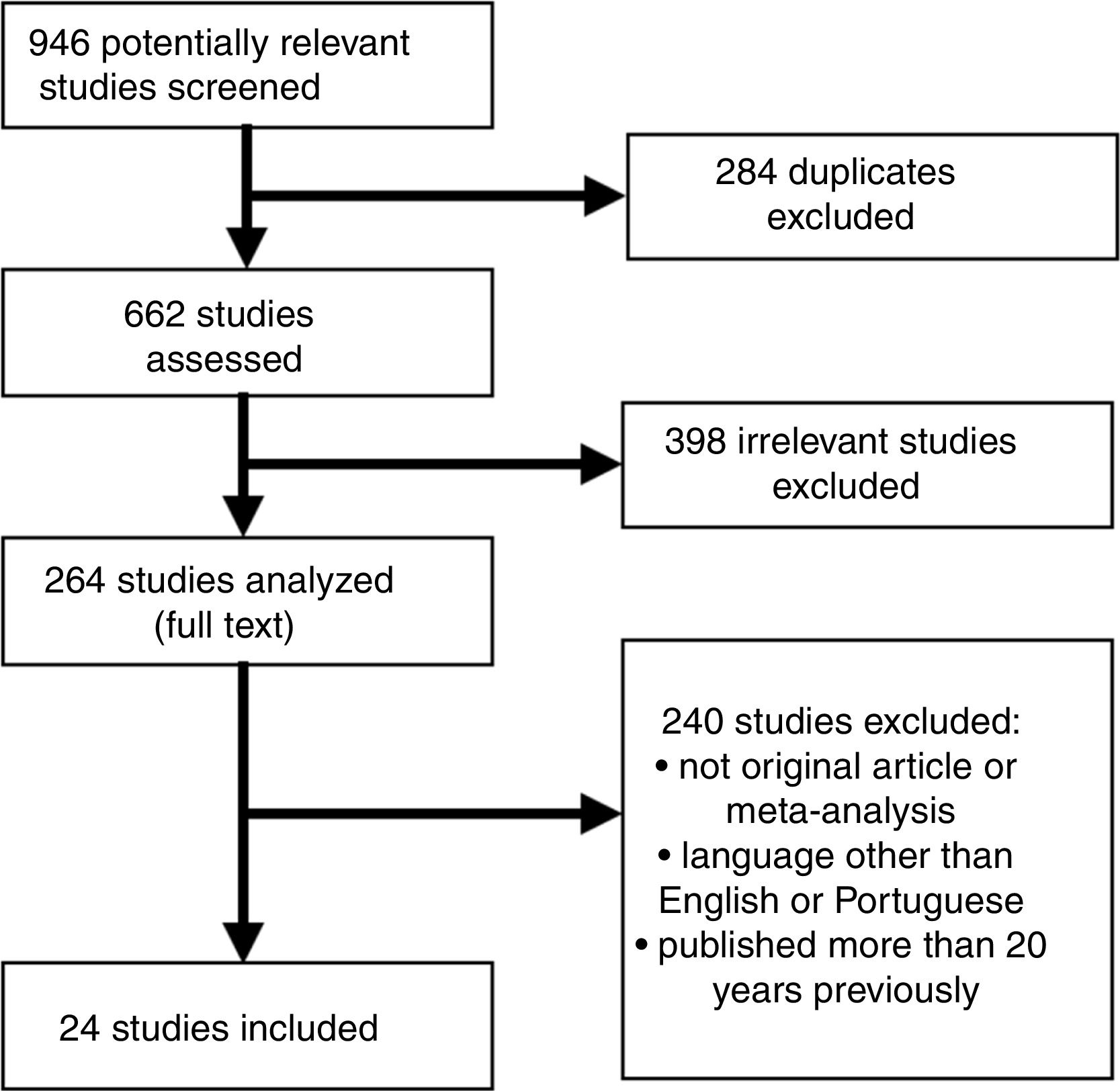
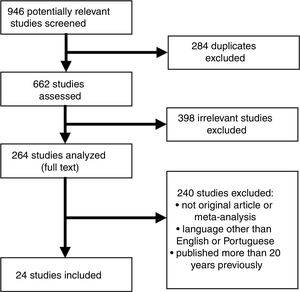
Subsequently, a PubMed search was carried out in the MEDLINE electronic database, using as keywords each biomarker to be studied with the descriptor (Descritores em Ciências da Saúde [DECS]/MeSH) “myocardial infarction”, and the keywords “coronary” and “cardiovascular”, using the Boolean operator “AND”. A total of 24 articles were found, consisting of original articles and reviews on biomarkers used in the context of ACS and MI, published in English between 2003 and 2017 (Figure 1).
Inclusion and exclusion criteriaThe inclusion criteria were: (a) animal or human studies; (b) biomarkers used in both ischemic heart disease and kidney disease; (c) original study or meta-analysis; (d) English or Portuguese language; (e) published in the previous 20 years. The exclusion criteria were: (a) duplicated studies; (b) commentaries or editorials. The articles were considered to be of high quality if they had been indexed in MEDLINE and published in journals with an acceptable impact factor.
Results and DiscussionAfter the articles were identified, an individual review of each biomarker and its current potential was carried out in the context of CAD.
Neutrophil gelatinase-associated lipocalinElevated levels of NGAL, a member of the lipocalin iron-carrier protein family, have been associated with ischemic and nephrotoxic kidney injury.3 It is measured using latex particles of sensitized anti-NGAL antibody, assessed in blood or urine samples, using validated kits. NGAL was assessed as a prognostic marker in 76 survivors of cardiac arrest, high levels indicating worse outcome, the optimal time point for predicting long-term outcomes being on the third day of the event. However, NGAL had a low predictive value alone, and according to the authors should be used together with other predictors of prognosis.4
Fibroblast growth factor 23FGF23 is a protein that participates in phosphate and vitamin D metabolism. It can be measured using an ELISA kit. In a cross-sectional study of 177 patients with CKD who underwent cardiac catheterization, FGF23 levels were shown to be associated with severity of CAD after adjusting for traditional risk factors.5
Another study analyzed 833 patients with stable CAD who had 402 events (death or cardiovascular event) during a median follow-up of six years. After adjustment for traditional risk factors, patients with levels in the highest tertile had a two-fold higher risk of death or cardiovascular events.6
Klotho is a co-receptor associated with FGF23. In a study of 956 patients, Klotho deficiency was associated with the occurrence of occult atherosclerotic disease.7 In another report, reduced Klotho gene expression in the aorta was also associated with CAD severity.
Tissue inhibitor of metalloproteinase-2TIMP-2 induces cell-cycle arrest. There have been few reports on the measurement of this biomarker in cardiovascular disease. TIMP-2 levels can be measured by NephroCheck® (Astute Medical), the commercial name of a combination of two urinary biomarkers, TIMP-2 and insulin-like growth factor-binding protein 7 (IGFBP7), expressed as [TIMP-2]·[IGFBP7], that can be used to identify patients at high risk of AKI. In 2014, the FDA approved marketing of NephroCheck® to measure urinary [TIMP-2]·[IGFBP7] and to determine if certain critically-ill patients are at risk of developing moderate to severe AKI. Regarding cardiovascular implications, studies on TIMP-2 are experimental and focus on the pathophysiology of angiogenesis and atherosclerosis. An experimental study showed that mice with genetic alterations in the TIMP2 gene (-/-) had decreased matrix metalloproteinase (MMP)-2 expression and increased anti-angiogenic factors, which resulted in abnormal ventricular remodeling and severe heart failure.8 Additional evidence to support the role of TIMP-2 in protecting against CAD comes from studies examining poly(ADP-ribose) polymerase (PARP) and apolipoprotein E (ApoE) genes. Mice with a genetic deficiency in PARP fed an atherogenic diet demonstrated an increase in TIMP-2 expression, counteracting the deleterious effects of MMP activation.9 A study in APOE -/- knockout mice demonstrated that TIMP-2 plays a greater protective role than TIMP-1 during the pathogenesis of atherosclerosis, in part by suppressing MMP-14-dependent monocyte/macrophage accumulation in atherosclerotic plaques.10 A study involving 67 patients with ACS (24 controls) concluded that increased TIMP-2 expression disturbs the equilibrium of the MMP/TIMP inhibition system and, as a consequence, contributes to atherosclerotic plaque destabilization and the occurrence of ACS.11
SyndecansSyndecans are an important family of cell surface heparan sulfate proteoglycans. Syndecan 1 (SDC1 or sCD138) can be measured using the sCD138 ELISA kit, a solid phase sandwich ELISA for the in-vitro qualitative and quantitative determination of sCD138 in supernatants, buffered solutions or serum and plasma samples. This assay will recognize both natural and recombinant human sCD138. In adult tissues, SDC1 is predominantly expressed by simple and stratified epithelia.12 In an experimental study with rats, SDC1 levels were elevated in rats with induced MI.12 Another experimental study demonstrated that increased SDC1 expression in MI protects against an excessive inflammatory response, reducing cardiac dilatation and ventricular dysfunction post-MI.13
Interleukin-6IL-6 is a 26-kDa pleiotropic immunomodulatory cytokine secreted by a variety of cell types that plays a critical role in many inflammatory processes. ELISA kits for the measurement of human or mouse IL-6 are available from a number of companies, although IL-6 is not usually measured in clinical practice. IL-6 is associated with atherosclerotic disease, as shown in a subanalysis of the STABILITY trial that studied 14 611 patients with stable chronic CAD and identified an association of IL-6 with the risk of major adverse cardiovascular events, cardiovascular death, MI, all-cause mortality, and hospital admission for heart failure.14 Serum IL-6>1 pg/ml in patients with chest pain and intermediate risk (according to the Atherosclerotic Cardiovascular Disease risk score) referred for coronary angiography was predictive of significant CAD.15 Measurement of IL-6 may be useful to reclassify intermediate risk patients into higher risk categories. Studies on IL-6 receptors suggest that IL-6 blockade could provide an innovative therapeutic approach to coronary heart disease prevention, but robust clinical trials and genetic testing in large populations are required to validate and prioritize new therapeutic targets.14,15
Galectin-3Studies have demonstrated the potential role of galectin-3, a protein in the lectin family, as a biomarker in patients with heart failure.16 It can be measured by quantitative sandwich enzyme immunoassay, for which kits have been approved by the FDA. For patients with CAD, elevated levels six hours post-MI were correlated with adverse clinical events at 30 days.17 Another study found no relationship between galectin-3 levels and cardiovascular events but did find an association with new-onset atrial fibrillation and new diagnosis of heart failure.18 The GIPS-III study analyzed 263 patients and found that galectin-3 was an independent predictor of left ventricular ejection fraction four months post-MI.19
Vascular cell adhesion molecules VCAM-1 and ICAM-1Plasma-soluble VCAM-1 and ICAM-1 can be measured using commercially available ELISA kits. A meta-analysis of 18 studies with 3546 cases found that the K469E polymorphism of the ICAM1 gene increases the risk of developing CAD.20 Another study involving this polymorphism and a second polymorphism, G2412, found no association between these genetic alterations and the occurrence of CAD and MI. In a subgroup of 2880 patients from the MESA study in whom ICAM-1 and VCAM-1 polymorphisms were analyzed, the authors found that ICAM-1 gene polymorphisms were associated with increased circulating ICAM-1 levels, but not with these patients’ coronary calcium score, exemplifying the conflicting results in the literature on these genetic polymorphisms.21 Another interesting study, which measured circulating ICAM-1 and VCAM-1 levels and assessed degree of restenosis in 15 patients with stable angina before and six months after bare-metal stent implantation, concluded that elevated circulating levels of VCAM-1, but not of ICAM-1, were correlated with stent restenosis.22 Circulating ICAM-1 and VCAM-1 levels were measured in a study that included 75 patients with CAD, in which only VCAM-1 was shown to be a powerful predictor of major events in patients with ACS.23 In a subgroup of the PRIME study in which patients who experienced an acute event during five-year follow-up were compared with controls, high ICAM-1 levels predicted future cardiac events. Elevated circulating ICAM-1, VCAM-1 and E-selectin levels have also been observed in patients with coronary slow-flow; these could be indicators of inflammation and endothelial activation.24,25
Study limitationsThe main limitation of this study is its methodology, which is a literature review. Further studies will certainly better establish the role of each novel biomarker in clinical practice, through large randomized clinical trials and meta-analyses. However, this study highlights the current literature suggesting that these biomarkers can be used in clinical settings and clinical studies.
ConclusionThere are several promising biomarkers that are potentially useful prognostic predictors in the context of CAD. FGF23, syndecan-1, IL-6 and galectin-3 are likely to play an important role in atherosclerosis, and in the future they may be used as diagnostic and prognostic biomarkers in this setting. Further studies are required to better investigate the patterns and correlations of each of these biomarkers with stable CAD and ACS. Based on our review, clinical applicability, time of collection, reference values, and association with relevant clinical outcomes are issues yet to be elucidated, reflecting the heterogeneity of articles in the current scientific literature.
FundingNo financial support needed.
Conflicts of interestThe authors have no conflicts of interest to declare.