Takotsubo syndrome (TTS) is characterized by transient left ventricular (LV) dysfunction, typically mimicking an anterior wall myocardial infarction (MI), without obstructive coronary artery disease. In the few published reports assessing myocardial deformation in TTS and MI, no consistent differences have been described between the two entities. We sought to characterize global and regional function in TTS and to compare it with a population with MI.
MethodsClinical data, including echocardiography, were gathered from 17 TTS patients and 20 anterior wall ST-segment elevation myocardial infarction (STEMI) controls. Peak systolic longitudinal strain was determined for each LV segment using speckle tracking imaging, and global and mean apical, midventricular and basal longitudinal strain were calculated from these.
ResultsBoth TTS and STEMI patients presented significant LV systolic dysfunction, and there were no significant differences in ejection fraction or global longitudinal strain. Regional longitudinal strain was more severely impaired in basal inferolateral and mid anterolateral segments in the TTS group and in apical anteroseptal segments in the STEMI group. Mean longitudinal strain was worse in the basal segments of TTS patients (-9.8±2.9 vs. -12.4±4.1%, p=0.010), with no significant differences in mid and apical segments. The basal/apical ratio was significantly lower in this group as well (1.51±0.86 vs. 2.94±1.88, p=0.006).
ConclusionsWhile both TTS and STEMI feature significantly impaired global systolic function, we found a regional pattern of worse basal longitudinal strain and a lower basal/apical ratio in the former. These suggest generalized myocardial impairment in TTS, providing new clues about its pathophysiology and possible specific echocardiographic changes.
A síndrome de Takotsubo (TTS) é caracterizada por disfunção ventricular esquerda transitória, habitualmente simulando um enfarte agudo do miocárdio (STEMI) anterior, sem doença coronária obstrutiva. Nos poucos trabalhos publicados avaliando deformação miocárdica não estão descritas diferenças consistentes entre as duas entidades. Procurámos caracterizar a função global e regional na TTS e compará-la com uma população com STEMI.
MétodosReunimos dados clínicos, incluindo ecocardiográficos, de 17 doentes com TTS e 20 controlos com STEMI anterior. O pico sistólico de strain longitudinal foi determinado para cada segmento ventricular usando speckle tracking; a partir destes calculou-se o strain longitudinal global e a média dos segmentos apicais, médios e basais.
ResultadosTanto os doentes com TTS como STEMI apresentaram disfunção sistólica ventricular esquerda importante, sem diferença significativa da fração de ejeção e strain longitudinal global. O strain regional foi pior nos segmentos basal inferolateral e médio anterolateral do grupo com TTS e no apical anteroseptal dos doentes com STEMI. O strain longitudinal médio dos segmentos basais foi pior nos doentes com TTS (-9,8±2,9 versus -12,4±4,1%, p=0,010), sem diferença significativa nos médios ou apicais. A razão basal/apical também foi mais baixa neste grupo (1,51±0,86 versus 2,94±1,88, p=0,006).
ConclusõesApesar de tanto a TTS como o STEMI apresentarem disfunção sistólica global importante, encontrámos um padrão de pior strain longitudinal basal e uma razão basal/apical mais baixa nos primeiros. Isto sugere uma disfunção miocárdica generalizada na TTS e fornece novas pistas relativamente à sua fisiopatologia e possíveis alterações ecocardiográficas específicas.
Takotsubo syndrome (TTS) typically presents with symptoms, electrocardiographic changes and mild elevation of cardiac enzymes that mimic myocardial infarction. It is characterized by transient left ventricular (LV) dysfunction extending beyond a single coronary territory, in the absence of obstructive coronary artery disease.1–3 The classic echocardiographic description is of apical akinesia, with or without mid-ventricular involvement, associated with basal hypercontractility; alternative patterns of regional dysfunction have been described, although less frequently.4,5 These features, which most frequently follow an emotional or physical trigger, were first described by Sato and colleagues in the 1990s.6 Initially believed to be a benign entity, it has been shown to be associated with non-negligible complications and an estimated 4-5% mortality.4,7–9 Its etiology is still somewhat elusive, but the most widely accepted hypotheses focus on catecholamine increase owing to sympathetic stimulation, which could cause myocardial dysfunction through adrenoceptor-mediated damage, direct toxicity, and endothelial dysfunction.10–12
Myocardial deformation analysis with speckle-tracking echocardiography has been shown to be more sensitive to lesser degrees of dysfunction than parameters such as ejection fraction, and is nowadays part of standard practice for detection of subclinical dysfunction in settings such as chemotherapy.13,14 Its use in TTS patients has been described in small reports, mainly focusing on serial assessment and possible persisting subclinical dysfunction.15–17 Some groups have also described abnormalities particular to this entity,18,19 however these findings have not been reproduced in larger studies.
Our goal was to characterize global and regional myocardial dysfunction in TTS using standard parameters as well as strain assessment with speckle-tracking echocardiography. A comparison with ST-segment elevation myocardial infarction (STEMI) sought to find patterns distinguishing between the two entities.
MethodsStudy populationWe retrospectively included 17 patients admitted to our hospital with a diagnosis of TTS. This was defined according to the Heart Failure Association diagnostic criteria,1 which include transient regional wall motion abnormalities in the absence of culprit atherosclerotic coronary artery disease on invasive angiography or other pathological conditions to explain the LV dysfunction; new and reversible electrocardiographic abnormalities, elevated serum natriuretic peptide and small elevation in cardiac troponin during the acute phase; and recovery of ventricular systolic function on cardiac imaging at follow-up. Predominantly basal and/or mid-ventricular involvement was an exclusion criterion.
A control group was formed of 20 randomly selected subjects with no prior history of coronary artery disease admitted to our department for anterior wall STEMI.
Besides the above criteria, inclusion in both groups required availability of transthoracic echocardiography acquisitions suitable for speckle-tracking strain assessment obtained within 48 hours of symptom onset.
EchocardiogramAll patients underwent comprehensive transthoracic echocardiographic examination after admission. Standard acquisitions were obtained using a 2.5 MHz phased-array transducer and either a Vivid 6 or Vivid 7 machine (GE Ultrasound). Post-processing of apical 4-, 2- and 3-chamber images at 50-80 frames per second was performed using the 2D strain module of the EchoPAC software (GE Ultrasound). Endocardial borders were defined from end-systolic frames, with further adjustments when necessary to optimize automated speckle tracking, according to published guidelines.13,20
Peak systolic longitudinal strain was determined for each myocardial segment using an 18-segment model, and global longitudinal strain was calculated as the mean of all segments. In addition, three LV slices – apical, midventricular and basal – were analyzed, and the mean of the peak systolic longitudinal strain in the six segments in each slice was calculated, from which the basal/apical ratio was derived.
Statistical analysisContinuous variables are presented as mean ± standard deviation or median [interquartile range]. Comparisons of continuous data were performed using the independent samples t test or Mann-Whitney U test, as appropriate. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. A p-value <0.05 was considered statistically significant.
All statistical analysis was performed using IBM SPSS Statistics version 22.
ResultsA total of 17 TTS patients were compared with 20 STEMI controls. Baseline demographic, clinical and laboratory features are summarized in Table 1. All TTS patients were female, with a mean age of 64 years (ranging from 33 to 84 years), while 19 of the STEMI patients were male, with mean age 57 years (37-76 years). The overall cardiovascular risk factor profile of the two groups was similar, except for current or previous smoking, which was more prevalent in STEMI than in TTS patients.
Demographic, clinical and laboratory features of the study sample.
| TTS | STEMI | p | |
|---|---|---|---|
| Age, yearsa | 64.5±11.9 | 57.2±9.5 | 0.048 |
| Femaleb | 17 (100%) | 1 (5.0%) | <0.001 |
| Hypertensionb | 12 (70.6%) | 12 (60.0%) | 0.50 |
| Diabetesb | 4 (23.5%) | 2 (10.0%) | 0.38 |
| Dyslipidemiab | 6 (35.3%) | 10 (50.0%) | 0.37 |
| Ever-smokersb | 1 (5.9%) | 14 (70.0%) | <0.001 |
| Peak troponin I, ng/lc | 2.6 [1.1-5.0] | 95.1 [65.7-174.6] | <0.001 |
| Peak BNP, pg/mlc | 546 [164-1185] | 53 [20-142] | <0.001 |
| BNP/troponin I ratioc | 310.1 [90.4-704.1] | 0.6 [0.2-2.2] | <0.001 |
| Neutrophils, ×109/lc | 7.9 [7.1-8.4] | 8.8 [6.2-11.1] | 0.56 |
| CRP, mg/lc | 5.2 [2.3-19.7] | 5.4 [1.8-13.1] | 0.75 |
| Albumin, g/lc | 38.0 [36.0-40.1] | 37.8 [36.5-40.4] | 0.83 |
BNP: brain natriuretic peptide; CRP: C-reactive protein; STEMI: ST-segment elevation myocardial infarction; TTS: Takotsubo syndrome.
In the TTS group, 11 patients developed symptoms after an emotional stressor, two after a physical stressor, and four had no identifiable trigger; 16 presented with primary TTS and only one with secondary TTS. The electrocardiogram at hospital admission showed ST-segment elevation in six patients, T-wave inversion in four, ST-segment depression in one and other nonspecific changes in the remaining six patients.
All STEMI patients underwent emergent coronary angiography which showed a culprit left anterior descending (LAD) artery lesion (11 in the proximal segment, eight mid and one distal), with single-vessel disease in 11 of them (55%). Primary angioplasty with stent implantation was performed in 18 patients (90%), the others being two cases of spontaneous reperfusion, one with no need for intervention and the other with three-vessel disease for deferred surgical revascularization.
Laboratory tests showed that peak troponin I levels were higher in STEMI patients and peak brain natriuretic peptide (BNP) was higher in TTS patients. The BNP/troponin I ratio was also significantly higher in the latter, as previously reported.21 By contrast, neutrophil count and acute-phase reactant levels were not significantly different.
Transthoracic echocardiography on admission showed significant LV systolic dysfunction in TTS and STEMI patients, and ejection fraction was similar in the two groups (Table 2). Diastolic function parameters were also comparable in patients in both groups.
Left ventricular systolic and diastolic function parameters obtained from baseline echocardiography.
| TTS | STEMI | p | |
|---|---|---|---|
| Ejection fraction, % | 37.8±5.5 | 36.9±7.1 | 0.66 |
| EDVI, ml/m2 | 61.6±8.4 | 62.6±9.7 | 0.75 |
| WMSI | 1.93±0.38 | 1.92±0.16 | 0.87 |
| E, cm/s | 71.2±22.3 | 65.6±16.5 | 0.39 |
| E/A | 1.10±0.64 | 1.00±0.38 | 0.56 |
| E/e’ | 10.7±3.8 | 9.9±2.3 | 0.50 |
| TR velocity, m/s | 2.5±0.3 | 2.4±0.2 | 0.56 |
| LA volume index, ml/m2 | 28.8±4.9 | 24.9±6.9 | 0.062 |
Values are mean ± standard deviation.
EDVI: end-diastolic volume index; LA: left atrial; STEMI: ST-segment elevation myocardial infarction; TR: tricuspid regurgitation; TTS: Takotsubo syndrome; WMSI: wall motion score index.
As with ejection fraction, LV global longitudinal strain was impaired in both groups, although with no statistically significant difference (-10.3±2.9 in TTS vs. -10.1±3.7% in STEMI, p=0.9).
Regarding regional function, longitudinal strain was worse in TTS patients in basal inferolateral and mid anterolateral segments and, conversely, worse in STEMI patients in the apical anteroseptal segment (Table 3).
Peak systolic regional longitudinal strain (%) by left ventricular segment.
| TTS | STEMI | p | |
|---|---|---|---|
| Basal segments | |||
| Anterior | -9.0±5.9 | -10.0±5.2 | 0.59 |
| Anteroseptal | -7.7±6.4 | -6.8±7.1 | 0.69 |
| Inferoseptal | -9.0±4.5 | -11.4±4.5 | 0.11 |
| Inferior | -13.3±6.8 | -16.6±5.6 | 0.12 |
| Inferolateral | -11.9±9.0 | -17.2±6.7 | 0.053 |
| Anterolateral | -10.7±6.3 | -14.9±5.6 | 0.041 |
| Mid-ventricular segments | |||
| Anterior | -6.6±5.5 | -7.2±4.0 | 0.72 |
| Anteroseptal | -8.7±5.9 | -6.5±5.7 | 0.26 |
| Inferoseptal | -10.5±4.4 | -9.0±4.6 | 0.34 |
| Inferior | -10.3±6.1 | -13.1±4.4 | 0.12 |
| Inferolateral | -8.4±5.6 | -12.3±4.7 | 0.031 |
| Anterolateral | -8.8±4.1 | -11.6±4.3 | 0.057 |
| Apical segments | |||
| Anterior | -4.5±6.2 | -2.9±7.5 | 0.52 |
| Anteroseptal | -8.4±6.5 | -5.1±6.6 | 0.15 |
| Inferoseptal | -11.0±7.1 | -6.3±6.4 | 0.039 |
| Inferior | -8.0±5.9 | -7.6±5.6 | 0.81 |
| Inferolateral | -6.6±5.5 | -6.0±6.2 | 0.78 |
| Anterolateral | -9.0±5.7 | -6.8±5.6 | 0.23 |
Values are mean ± standard deviation.
STEMI: ST-segment elevation myocardial infarction; TTS: Takotsubo syndrome.
Concerning the three LV slices, mean mid and apical longitudinal strain did not differ significantly between the two groups (Table 4). Basal longitudinal strain, however, was worse in TTS than in STEMI patients (-9.8±2.9 vs. -12.4±4.1%, p=0.010).
Mean peak systolic longitudinal strain (%) in the six basal, mid and apical left ventricular segments.
| TTS | STEMI | p | |
|---|---|---|---|
| Basal segments | -9.8±2.8 | -12.4±3.1 | 0.010 |
| Mid-ventricular segments | -8.9±2.8 | -9.9±3.1 | 0.328 |
| Apical segments | -8.4±5.2 | -6.0±4.3 | 0.134 |
Values are mean ± standard deviation.
STEMI: ST-segment elevation myocardial infarction; TTS: Takotsubo syndrome.
Analyzing the differences in dysfunction throughout the LV axis, the ratio between mean longitudinal strain in the basal and apical slices was significantly lower in TTS patients as well (1.51±0.86 vs. 2.94±1.88, p=0.006).
A subanalysis comparing TTS and STEMI patients with non-proximal LAD culprit lesions showed similar results, with worse basal longitudinal strain (-9.8±2.9 vs. -13.9±2.7%, p=0.002) and lower basal/apical ratio in the former (1.51±0.86 vs. 3.23±2.51, p=0.016).
A trend towards worse basal longitudinal strain was also seen in TTS compared to STEMI patients with proximal LAD culprit lesions, although this did not reach statistical significance (-9.8±2.9 vs. -11.3±3.0%, p=0.19). By contrast, proximal LAD patients had worse basal longitudinal strain than TTS (-8.4±5.2 vs. -4.9±2.2, p=0.046); moreover, this resulted in a still statistically significant lower basal/apical ratio in TTS patients (1.51±0.86 vs. 2.70±1.23, p=0.005).
DiscussionOur study shows that, while LV dysfunction is obviously a key feature in both TTS and myocardial infarction patients, the former have worse mean longitudinal strain in basal segments. This finding challenges the eyeball appearance of basal hypercontractility, possibly related to the contrasting markedly akinetic mid and apical segments with a ballooning appearance. The basal strain impairment could be explained, first of all, by unfavorable loading effects and geometrical changes due to remote dysfunctional segments, as has been described in acute myocardial infarction.22 Additionally, the trend toward worse longitudinal strain in TTS patients in inferior, inferolateral and anterolateral segments, similar to the findings of Heggemann et al.,18 suggests more global myocardial dysfunction, as would be expected considering the different pathophysiological mechanisms. Still, we would be inclined to interpret these segmental results with caution, due not only to the number of patients but also to the statistical limitations of using multiple comparisons.
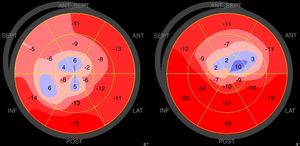
The difference between the two groups lost significance when STEMI patients with proximal LAD culprit lesions only were analyzed, which could be due to the limited sample size, as the numerical difference persisted. Nevertheless, the resulting lower basal/apical longitudinal strain ratio in TTS remained statistically significant both in the entire sample and in each STEMI subgroup, translating into a smaller ‘dysfunction gradient’, providing further evidence of more generalized myocardial impairment in these patients (Figure 1).
Unlike previous studies, we did not identify worse global LV systolic function in TTS than in STEMI patients as assessed by ejection fraction or global longitudinal strain.18,23 This might help explain why we did not find the more severe midventricular and apical impairment in TTS described by the same authors; nevertheless, this could also be an indicator of better comparability between the two groups, and thus increase confidence in the differences found. Neither do our findings support the existence of the ‘evil eye’ pattern of symmetrical apical involvement in TTS described by Sosa and Banchs.19 On the contrary, there was a trend towards worse longitudinal strain in all apical segments in STEMI patients.
Some limitations in our work should be acknowledged, first of all those inherent to any study with a retrospective design. Additionally, owing to the inclusion criteria used, our findings are only valid for classical TTS. The lack of gender matching is self-evident, but it is closely linked to the contrasting epidemiology of these diseases. The relatively small sample is also a limitation, as previously stated, that is related not only to the relative rarity of this diagnosis but also to the need to use information on patients whose echocardiograms were obtained from only one manufacturer, as speckle-tracking strain analysis is still not fully standardized across different platforms. Nevertheless, we believe these limitations are overcome by the importance of adding to knowledge on a disease about which our understanding still has important gaps.
One of the ultimate goals in TTS research is the ability to quickly and safely distinguish it from STEMI, thus enabling appropriate decisions on the appropriate timing for coronary angiography. Echocardiography is a readily accessible tool in clinical practice and, with appropriately validated specific features, would be the ideal means to this end. Right now, we believe that identifying potential parameters of interest and assessing them in different settings, as we sought to do, is essential for this objective, and will enable adequately powered prospective studies to be performed in the near future. Moreover, the identification of such distinctive characteristics is of great value in advancing our knowledge on the pathophysiology of TTS, which in turn is inextricably linked to research into new therapeutic strategies.
ConclusionsOur main findings are the worse mean longitudinal strain in basal LV segments of TTS compared with STEMI patients, and the smaller basal/apical longitudinal strain ratio in the former group. This smaller ‘dysfunction gradient’ compared to the localized phenomenon that is coronary artery disease is indicative of more generalized myocardial impairment than initially realized. If confirmed by further studies, it could help identify echocardiographic patterns specific to TTS and enhance both our knowledge of this disease and the clinical approach.
Conflicts of interestThe authors have no conflicts of interest to declare.