Multivessel disease in ST‐elevation myocardial infarction (STEMI) is associated with a worse prognosis. A multivessel approach at the time of primary percutaneous coronary intervention (PCI) is the subject of debate.
ObjectiveTo assess the impact of a multivessel approach on in‐hospital morbidity and mortality in patients with STEMI undergoing primary PCI.
MethodsWe studied patients from the Portuguese Registry of Acute Coronary Syndromes with STEMI and multivessel disease who underwent primary PCI. The 257 patients were divided into two groups: those who underwent PCI of the culprit artery only and those who underwent multivessel PCI. Cardiovascular risk factors, STEMI location, in‐hospital treatment, number and type of diseased and treated arteries, type of stent implanted and ejection fraction were recorded. The primary end‐point was defined as in‐hospital mortality and the secondary end‐point as the presence of at least one of the following complications: major bleeding, need for transfusion, invasive ventilation, heart failure and reinfarction.
ResultsMultivessel disease was found in 43.3% of the study population and a multivessel approach was adopted in 19.2% of these patients. There were no differences between the groups in cardiovascular risk factors or electrocardiographic presentation of STEMI. Patients undergoing multivessel PCI were more likely to be treated with drug‐eluting stents and glycoprotein IIb/IIIa inhibitors, and less likely to receive heparin therapy. There were no differences between the groups with regard to in‐hospital mortality or the incidence of complications.
ConclusionIn our population of patients with STEMI, a multivessel approach appears to be safe and not associated with increased in‐hospital mortality or morbidity.
A presença de doença coronária multivaso (DCM) no enfarte agudo do miocárdio com elevação de ST (EAMCST) associa‐se a um pior prognóstico. Atualmente, a sua abordagem no momento da angioplastia primária permanece motivo de controvérsia.
ObjetivoAvaliar o impacto da abordagem da DCM na morbilidade e mortalidade intra‐hospitalares em doentes com EAMCST submetidos a angioplastia primária.
MétodosDo Registo Nacional de Síndromes Coronárias Agudas estudaram‐se os doentes com EAMCST submetidos a angioplastia primária e que apresentassem DC em mais de uma artéria. Dos 257 doentes consideraram‐se dois grupos: doentes com angioplastia apenas da artéria culpada e doentes com angioplastia multivaso. Registaram‐se fatores de risco cardiovascular (FRCV), localização do EAM, pressão arterial sistólica, frequência cardíaca, tempo sintomas‐reperfusão, terapêutica no internamento, número, tipo de artérias com lesão e tratadas, tipo de stent e fração de ejeção. Avaliou‐se como endpoint primário a mortalidade intra‐hospitalar e como endpoint secundário a presença de pelo menos uma das complicações: hemorragia major, necessidade de transfusão, ventilação mecânica, insuficiência cardíaca e reenfarte.
ResultadosA presença de DCM foi encontrada em 43,3%. A abordagem multivaso foi efetuada em 19,2% destes doentes. Nos dois grupos não existiram diferenças nos FRCV e na apresentação eletrocardiográfica do EAM. Os doentes com angioplastia multivaso apresentaram maior taxa de colocação de stents revestidos por fármaco, mais terapêutica com inibidores das glicoproteínas IIb/IIIa e menos terapêutica com heparina. Entre os grupos não se registaram diferenças nos dois endpoints.
ConclusãoNa nossa população a abordagem multivaso no EAMCST parece constituir uma estratégia segura, não se associando a aumento da mortalidade e/ou morbilidade intra‐hospitalares.
Multivessel disease (MVD) is found in 40–65% of patients with ST‐segment myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) and is associated with a worse prognosis.1,2
However, the latest European Society of Cardiology (ESC) guidelines on STEMI limit multivessel PCI to cases of cardiogenic shock and recommend ischemia testing before PCI of non‐culprit vessels,3 but the data to support this safety concern are scant and studies and meta‐analyses examining strategies for MVD in STEMI continue to give conflicting results.4,5
A staged revascularization approach, with initial PCI of the culprit artery and subsequent revascularization of non‐culprit vessels is associated with lower mortality in STEMI patients with MVD compared to primary PCI of the culprit vessel and all other arteries with significant lesions.6,7 On the other hand, a registry and meta‐analysis including 61 764 STEMI patients with MVD showed that a multivessel revascularization strategy at the time of culprit lesion PCI is safe and is associated with a reduced rate of revascularization.4
Given the lack of evidence for the optimal approach to primary PCI in MVD, the aim of this study was to assess the impact of a multivessel approach on in‐hospital morbidity and mortality in patients with STEMI undergoing primary PCI included in the Portuguese Society of Cardiology's Registry of Acute Coronary Syndromes (ACS).
MethodsWe analyzed all patients with STEMI in the ACS Registry who underwent primary PCI between October 1, 2010 and October 31, 2011 (n=921) and who presented MVD, defined as ≥50% stenosis (n=399). Patients who underwent PCI of a non‐culprit vessel in a second procedure (n=132) were excluded, as were those with a history of coronary artery bypass surgery (n=10).
A total of 257 patients were included, who were divided into two groups: group 1, who underwent PCI of the culprit artery only (n=180) and group 2, those who underwent multivessel PCI (n=77). Demographic data, cardiovascular risk factors, STEMI location as defined in the ACS Registry (anterior myocardial infarction [MI], inferior MI, or MI with new‐onset left bundle branch block [LBBB]), systolic blood pressure and heart rate at admission, pain‐to‐balloon time, in‐hospital treatment, number and type of diseased and treated arteries, percentage of drug‐eluting stents, and ejection fraction were recorded.
The primary end‐point was defined as in‐hospital mortality and the secondary end‐point as the presence of at least one of the following complications: major bleeding as defined by the GUSTO criteria, need for transfusion, invasive ventilation, heart failure and reinfarction.
Statistical analysisContinuous variables were presented as means ± standard deviation and compared using the Student's t test, while categorical variables, expressed as relative frequencies, were compared with the chi‐square test or Fisher's exact test. A p value of <0.05 was considered statistically significant.
Multivariate analysis was performed to identify independent predictors of both endpoints.
ResultsCharacteristics of the study populationOf the patients who underwent primary PCI, 43.3% had MVD. Of these, 45.1% underwent PCI of the culprit vessel only, in 35.7% the culprit vessel was treated first and non‐infarct arteries later in the same hospitalization, and 19.2% underwent multivessel PCI.
The characteristics of the two study groups are described in Table 1, which shows that a higher proportion of patients were male in both groups, and that hypertension and dyslipidemia were the most common cardiovascular risk factors, being found in 70% and 60% of patients, respectively; no statistically significant differences between the groups were found in cardiovascular risk factors. Chronic renal failure was defined according to the criteria used in the ACS Registry (creatinine prior to admission >2.0 mg/dl), and left ventricular dysfunction was defined as ejection fraction <50%.
Characteristics of the study population.
| Group 1 (culprit vessel PCI only) n=180 | Group 2 (multivessel PCI) n=77 | p | |
| Age, years (mean ± SD) | 65.8±12.6 | 63.1±11.1 | NS |
| Male, n (%) | 135 (75.0) | 63 (81.8) | NS |
| CV risk factors, n (%) | |||
| Hypertension | 125 (69.5) | 53 (68.9) | NS |
| Diabetes | 49 (27.3) | 13 (16.2) | NS |
| Dyslipidemia | 105 (58.3) | 43 (56.1) | NS |
| Smoking | 54 (29.8) | 30 (38.2) | NS |
| Family history of CAD | 14 (7.6) | 8 (10.2) | NS |
| CRF | 6 (3.4) | 4 (5.3) | NS |
| ECG presentation, n (%) | |||
| Anterior MI | 85 (47.2) | 38 (49.4) | NS |
| Inferior MI | 94 (52.2) | 39 (50.6) | NS |
| New‐onset LBBB | 1 (0.6) | 0 (0.0) | NS |
| CAD, n (%) | |||
| 3 vessels+LM | 8 (4.1) | 2 (3.0) | NS |
| 3 vessels | 57 (31.8) | 21 (27.3) | <0.001 |
| 2 vessels+LM | 4 (2.3) | 0 (0.0) | NS |
| 2 vessels | 111 (61.8) | 54 (69.7) | <0.001 |
| Normal LVF, n (%) | 81 (44.7) | 48 (62.7) | 0.006 |
| SBP (mean ± SD), mmHg | 130.4±11.1 | 129.2±10.8 | NS |
| HR (mean ± SD), bpm | 78.9±12.1 | 77.9±10.9 | NS |
| Median pain‐to‐balloon time, min | 208 | 206 | NS |
CAD: coronary artery disease; CRF: chronic renal failure; CV: cardiovascular; HR: heart rate; LM: left main coronary artery; LBBB: left bundle branch block; LVF: left ventricular function; MI: myocardial infarction; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; SD: standard deviation.
There were no statistically significant differences between the groups in terms of ECG presentation, with a similar distribution of inferior and anterior MI.
At the time of primary PCI, patients in group 1 were more likely to present 3‐vessel disease and less likely to present 2‐vessel disease; there was no difference in the prevalence of 2‐ or 3‐vessel plus left main disease.
Patients in group 2 had a higher prevalence of normal left ventricular function (62.7 vs. 44.7%; p=0.006).
There were no significant differences between the groups in mean systolic blood pressure or heart rate at admission, or in median pain‐to‐balloon time.
Pharmacological therapyTable 2 shows pharmacological therapy during hospitalization. With the exception of heparin (more frequent in group 1) and glycoprotein IIb/IIIa inhibitors (more frequent in group 2), there were no significant differences between the groups. A high percentage of patients were medicated with aspirin and clopidogrel (100% in both cases) as well as statins, angiotensin‐converting enzyme (ACE) inhibitors and beta‐blockers.
Pharmacological therapy.
| Group 1 (culprit vessel PCI only)n=180 | Group 2 (multivessel PCI)n=77 | p | |
| Aspirin, n (%) | 180 (100) | 77 (100) | NS |
| Clopidogrel, n (%) | 180 (100) | 77 (100) | NS |
| Heparin, n (%) | 124 (68.7) | 23 (29.9) | <0.001 |
| Enoxaparin, n (%) | 78 (43.3) | 35 (45.5) | NS |
| Fondaparinux, n (%) | 9 (5.1) | 4 (5.2) | NS |
| Bivalirudin, n (%) | 1 (0.6) | 0 (0.0) | NS |
| Gp IIb/IIIa inhibitors, n (%) | 70 (38.8) | 57 (73.7) | <0.001 |
| ACE inhibitors, n (%) | 143 (79.3) | 64 (83.1) | NS |
| ARBs, n (%) | 4 (2.2) | 0 (0.0) | NS |
| Beta‐blockers, n (%) | 139 (77.1) | 64 (83.1) | NS |
| Statins, n (%) | 170 (94.4) | 75 (97.4) | NS |
| Diuretics, n (%) | 63 (35.0) | 21 (27.3) | NS |
ACE: angiotensin‐converting enzyme; ARBs: angiotensin receptor blockers; Gp: glycoprotein; PCI: percutaneous coronary intervention.
The complexity of coronary artery disease was similar in the two groups (Table 3), with the exception of the total number of lesions treated (higher in group 2) and in the type of vessels treated. Procedural success (defined as in the ACS Registry as <30% residual stenosis and TIMI flow 3 after PCI) was similar in the two groups, with a success rate of over 90%.
Coronary artery disease and invasive treatment.
| Group 1 (culprit vessel PCI only)n=180 | Group 2 (multivessel PCI)n=77 | p | |
| Diseased vessels (mean) | 2.4 | 2.3 | NS |
| Vessels treated, n (%) | |||
| LAD | 87 (48.3) | 59 (76.6) | <0.0001 |
| Cx | 22 (12.2) | 46 (59.7) | <0.0001 |
| RCA | 70 (38.9) | 57 (74.0) | <0.0001 |
| LM | 1 (0.6) | 2 (2.6) | NS |
| Total vessels treated (mean) | 1 | 2.1 | <0.0001 |
| Procedural success, n (%) | 176 (97.8) | 72 (93.5) | NS |
| Stenting (%) | 93.9 | 98.7 | NS |
| Stents per lesion (mean) | 1.1 | 1.4 | <0.0001 |
| Drug‐eluting stents (%) | 40.8 | 73.7 | 0.003 |
| Bare‐metal stents (%) | 59.2 | 26.3 | <0.001 |
Cx: circumflex artery; LAD: left anterior descending artery; LM: left main coronary artery; PCI: percutaneous coronary intervention; RCA: right coronary artery.
With regard to stenting, at least one stent was implanted in over 90% of the study population. Drug‐eluting stents were used more frequently in group 2 and bare‐metal stents more frequently in group 1.
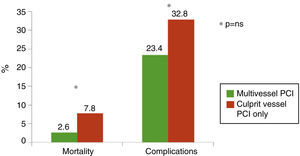
In‐hospital complications and mortalityOverall mortality was 5.2%. The primary and secondary endpoints of in‐hospital mortality and complications were slightly less frequent in patients undergoing multivessel PCI (Figure 1).
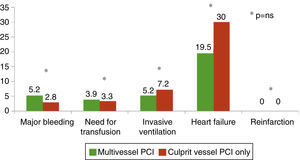
Figure 2 shows that heart failure was the most common complication in both groups, while the prevalences of major bleeding, need for transfusion and invasive ventilation were similar. There were no cases of reinfarction in either group. With the exception of a non‐significantly higher prevalence of heart failure in patients undergoing culprit vessel PCI only, the distribution of complications was similar in both groups.
Multivariate analysis (Figure 3) revealed that the independent predictors of in‐hospital mortality were left ventricular dysfunction (ejection fraction <30%), baseline creatinine ≥1.485 mg/dl and major bleeding during hospitalization. There was a non‐significant trend (p=0.09) for higher in‐hospital mortality in group 1.
Multivariate analysis was also performed to identify independent predictors of the secondary endpoint (at least one complication) and of the combined endpoint (primary and secondary endpoints), which showed that neither culprit vessel PCI only nor multivessel PCI was an independent predictor.
DiscussionThis study at the national level, which included various centers in Portugal, set out to analyze the impact of a multivessel approach in a real‐world population of STEMI patients undergoing PCI. The fact that the study population was taken from the ACS Registry, the inclusion criteria for which are clearly defined and applied equally to all centers, means that the same clinical data were available for all patients and could thus be analyzed in the same way.
MVD is common in STEMI patients undergoing primary PCI and is associated with higher morbidity and mortality. The prevalence of MVD in our study was 43.3%, a similar figure to that found in the EUROTRANSFER Registry and a South Korean registry, in which the prevalence was 51.5% and 47.9%, respectively.2,8
Despite the implications for prognosis, the ESC guidelines on STEMI and on myocardial revascularization continue to recommend multivessel PCI in STEMI only for patients with refractory heart failure and/or cardiogenic shock. However, in the real world, various registries show that a multivessel approach is adopted in between 9% and 24.4% of cases.2,4,9 In the Portuguese ACS Registry, this figure was 19.2%.
According to some authors, this discrepancy between the guidelines and clinical practice is the result of a lack of clinical evidence; the subject continues to arouse debate, which can only be resolved by a large international multicenter trial.
In our study, we set out to assess the impact of a multivessel approach on in‐hospital morbidity and mortality in patients with STEMI undergoing primary PCI.
The patients were divided into two groups: group 1, who underwent PCI of the culprit artery only and group 2, those who underwent multivessel PCI. The groups were similar in baseline clinical characteristics, with no significant differences in age, gender, cardiovascular risk factors or ECG presentation of MI (Table 1). The majority of patients in both groups were male, reflecting the higher prevalence of cardiovascular disease in men.
Hypertension was the most common cardiovascular risk factor, found in around 70% of patients, reflecting the high prevalence of hypertension in Portugal. Dyslipidemia was the second most frequent risk factor, while the percentages for smoking and diabetes were similar.
Angiography revealed that patients in group 1 had a slightly higher prevalence of 3‐vessel disease than in group 2 (31.8% vs. 27.3%; p<0.001). This may be explained by the fact that, although complete revascularization of 3‐vessel disease may improve prognosis, it is also associated with longer procedure times, increased contrast use, and greater risk of periprocedural MI.5 No significant differences in left main disease were found between the groups.
Pharmacological therapy (Table 2) followed the ESC guidelines for STEMI, with 100% of patients receiving aspirin and clopidogrel, around 95% receiving statins and around 80% receiving ACE inhibitors or angiotensin receptor blockers and beta‐blockers. Except for heparin (used more in group 1) and glycoprotein IIb/IIIa inhibitors (used more in group 2), there were no significant differences between the groups. The use of glycoprotein IIb/IIIa inhibitors in STEMI patients was the subject of a large meta‐analysis by de Luca et al., which concluded that the benefits were greater in higher‐risk patients.10 This may explain their higher rate of use in more complex procedures such as multivessel PCI.
Traditionally, drug‐eluting stents have been associated with lower restenosis and revascularization rates and improved survival in STEMI patients.11–14 This may explain why they were used more frequently in patients undergoing multivessel PCI than in those undergoing culprit artery PCI only, in whom bare‐metal stents were more common.
No significant differences were seen in our population in either the primary (in‐hospital mortality) or secondary (any of the defined complications) endpoints. However, those undergoing multivessel PCI had a higher prevalence of normal left ventricular function during hospitalization (62.7% vs. 44.7%; p=0.006). The significance of this finding is unclear, since the ACS Registry data do not specify whether patients’ ejection fraction was assessed before or immediately after PCI or before discharge.
The quest for the optimal strategy for MVD in STEMI patients remains the subject of debate.5 In a retrospective study, Hannan et al. compared mortality in‐hospital and at 12, 24, and 42 months in patients who underwent culprit vessel PCI only or multivessel PCI in the index procedure or subsequently, and concluded that culprit vessel PCI was associated with lower in‐hospital mortality and staged multivessel PCI was associated with lower 12‐month mortality.7 Similarly, a meta‐analysis of four prospective and 14 retrospective studies including a total of 40 280 patients concluded that a staged approach to MVD (primary PCI of the culprit vessel and PCI of non‐culprit vessels in subsequent procedures) was associated with lower mortality than primary PCI of the culprit vessel only and that simultaneous multivessel PCI resulted in the highest short‐ and long‐term mortality.6 The conclusions of both these studies support the recommendations in the ESC guidelines on STEMI and on myocardial revascularization.
On the other hand, the South Korean registry revealed no significant differences in mortality, MI or revascularization at one year between patients undergoing culprit vessel PCI only or multivessel PCI.8 Similar conclusions had previously been drawn in a small prospective trial by Khattab et al.,15 while a meta‐analysis by Bengalore et al. involving 61 764 patients found no significant differences in short‐term mortality, MI or stroke or in long‐term MI, stent thrombosis or target vessel revascularization, the authors concluding that multivessel PCI appears to be safe compared to culprit artery‐only revascularization.4
As in this meta‐analysis and the Korean registry, in our study there were no differences between the groups in mortality or the incidence of complications.
Since the existing studies and meta‐analyses on a multivessel approach to STEMI draw conflicting conclusions, there is a need for large randomized multicenter trials to clarify the question, for which our study may provide a further stimulus.
LimitationsThis study has the limitations inherent to all registry‐based analyses. The ACS Registry is voluntary, and so not all centers in Portugal participate and participating centers do not necessarily include all their patients; as a result the sample size is small, which reduces the statistical validity of some of the differences between the groups.
Another limitation derives from the format of the ACS Registry, in which the criteria used by different operators to decide between culprit vessel PCI only and multivessel PCI are not specified, which also weakens the validity of some of the results obtained.
ConclusionsIn our population of STEMI patients undergoing primary PCI, the prevalence of MVD was 43.3%, and a simultaneous multivessel approach was adopted in 19.2%. A multivessel approach appears to be a safe alternative to conventional culprit‐vessel only PCI, with a non‐significant tendency for lower in‐hospital morbidity and mortality.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestsThe authors have no conflicts of interest to declare.
Registo Nacional de Síndromes Coronárias Agudas da Sociedade Portuguesa de Cardiologia.
Please cite this article as: Santos AR, Piçarra BC, Celeiro M, et al. Abordagem multivaso no enfarte agudo do miocárdio com elevação do segmento ST: impacto na morbilidade e mortalidade intra‐hospitalares. Rev Port Cardiol. 2014;33:67–73.