Heart failure (HF) represents a huge financial and economic burden worldwide. Some authors advocate that remote monitoring should be implemented to improve HF management, but given its increasing incidence, as well as its morbidity and mortality, a question still remains: are we monitoring it properly? There is no shortage of literature on home monitoring devices, however, most of them are designed to monitor an unsuitable array of variables and, to the best of our knowledge, there are no large randomized studies about their impact on morbidity/mortality of HF patients.
ObjectiveDescription of a novel monitoring device.
MethodsAs a solution, we designed MONITORIA (MOnitoring NonInvasively To Overcome mortality Rates of heart Insufficiency on Ambulatory).
ResultsThis is a multimodal device that will provide real time monitoring of vital, electrophysiological, hemodynamic and chemical signs, transthoracic impedance, and physical activity levels. The device is meant to perform continuous analysis and transmission of all data. Significant alterations in a patient's variable will alert the attending physician and, in case of potentially life-threatening situations, the national emergency medical system. The MONITORIA device will, also, have a function that sends shocks or functions as a pacemaker to treat certain arrhythmias/blockades. This function can be activated the very first time the patient utilizes it, based on their risk of sudden cardiac death.
Discussion/ConclusionsMONITORIA is a promising device mostly because it is included in a follow-up program that takes into account a multi-perspective feature of HF development and is based on the real world patient, adapting innovations not to the disease but rather to the patients.
A insuficiência cardíaca (IC) acarreta sobrecarga económico-financeira, mundialmente, e alguns autores advogam a implantação de telemonitorização no acompanhamento desta doença. Contudo, tendo em conta a crescente incidência e morbimortalidade, impõe-se uma questão: estaremos nós a monitorar corretamente a IC? A literatura é profícua em dispositivos de monitoração ambulatória. Todavia, a maioria assenta numa matriz inadequada de variáveis e, até agora, ainda não foram conduzidos grandes ensaios clínicos randomizados e controlados acerca do seu impacto na morbimortalidade dos doentes com IC.
ObjectivoDescrição de um novo dispositivo de monitoração.
MétodosDeste modo, nós desenhamos o MONITORIA (MOnitoring NonInvasively To Overcome mortality Rates of heart Insufficiency on Ambulatory) por forma a colmatar esta lacuna.
ResultadosO MONITORIA é um dispositivo multimodal que permitirá a monitoração em tempo real de variáveis vitais, eletrofisiológicas, hemodinâmicas e químicas, impedância transtorácica e níveis de actividade física. O dispositivo está programado para realizar análise e transmissão contínuas. Face a alterações significativas numa determinada variável, o sistema emitirá um alerta para o médico responsável e, caso se trate de uma situação de perigo de vida, para o instituto nacional de emergência médica (INEM). Num futuro próximo, o MONITORIA disporá também de desfibrilhador/pacemaker para tratamento de certas arritmias/bloqueios. Esta propriedade poderá ser ativada quando da sua primeira utilização, em função do risco de morte súbita.
Discussão/conclusãoTrata-se de um dispositivo promissor sobretudo porque se insere num programa de follow-up que tem em consideração uma perspetiva multifacetada do desenvolvimento da IC e se baseia no doente real, adaptando a inovação ao doente em detrimento da doença.
Heart failure (HF) is a multifactorial chronic syndrome with an enormous public health impact.1 Its incidence is also increasing, as are the morbidity and mortality rates, despite the latest therapeutic innovations.
For this reason, it is perfectly permissible to speculate about the unsuitable way we are monitoring HF in the patient's home. In fact, perhaps we have not been monitoring it properly so far, predicting it in a timely or sensitive enough manner, or even treating the correct decompensating factors.
We performed an exhaustive literature search on this topic and found that increases in heart rate,2 low heart rate variability, a rise in ventricular filling pressures, atrial and ventricular arrhythmias, high respiratory rate and pulmonary congestion are the main parameters believed to predict precipitating HF decompensations.3,4
HF home monitoring has been shown to improve HF management and ambulatory follow-up of HF patients has proven to be cost-effective.5,6
As we have discussed in part I of this series of articles, in which we presented the theoretical rationale for the development of MONITORIA, home monitoring of HF should be by means of a device as this is the only way we can obtain continuous objective data on patient status, apart from the healthcare provider visits and self-monitoring.7
Telemonitoring without implantable devices is perhaps the best option for elderly advanced patients, and improved technology and effective predictive models would anticipate sudden decompensation events. Accordingly, we designed a non-invasive device that we believe will revolutionize outpatient follow-up, management of HF and its outcomes.
This device (designated from now on as MONITORIA) will provide non-invasive remote monitoring of HF patients, by capturing vital and electrophysiological signs (heart rate, and heart rate variability, T wave variability, electric conduction abnormalities, atrial and ventricular arrhythmias, respiratory rate and peripheral oxygen saturation), hemodynamic signs (systemic arterial blood pressure, right and left atrial pressures), transthoracic bioimpedance), chemical signs (skin sodium content), and physical activity levels.
The MONITORIA device (MOnitoring NonInvasively To Overcome mortality Rates of heart Insufficiency on Ambulatory), currently under development and being tested, is a state-of-the-art multimodal array of sensors that will provide real-time monitoring of several physiologic, hemodynamic and chemical variables in HF patients, allowing health professionals to have a timely multifaceted perspective of HF.
By providing these data, it will be possible to detect more promptly early signs of decompensation, act on them and stabilize patients at home, avoiding hospitalizations. Furthermore, any time the patient needs a medical emergency team (MET) at home (such as in the event of an effective or impending cardiac arrest or suspicion of a myocardial infarction) to overcome possible waste of time (sometimes the MET can take upwards of 30 min to reach the patient), MONITORIA is designed with a cardioverter-defibrillator function. This way, in certain cases of cardiac arrest (including the presence of ventricular fibrillation or sustained ventricular tachycardia), the device will send a shock trying to defibrillate the patient, while waiting for the emergency team to arrive.
ObjectivesThe objective of our researchis to develop the most appropriate device for home monitoring of chronic HF. This device is MONITORIA and for simplicity we chose to separate our MONITORIA research into three articles. This is part II, and the purpose is to describe the technical specifications of the device's design. Part I gave theoretical basis and rationale surrounding MONITORIA's development. Part III will present the MONITORIA prototype and the validation tests.
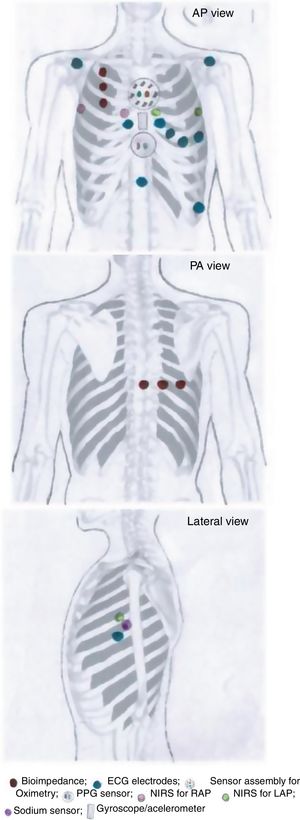
MONITORIA: Sensor array designTaking into account the rationale presented in the previous section, supported by evidence in the literature, MONITORIA is being designed with the set of sensors/electrodes shown in Figure 1, which are described in detail below.
Transthoracic impedance sensor systemMONITORIA will capture transthoracic impedance using six standard electrocardiogram (ECG) electrodes, similar to those used by Shochat et al.:8 three placed vertically on the right side of the chest, 4.5 cm from midline, with the upper electrode attached precisely under the clavicle; and an additional set of three electrodes placed dorsally, along a horizontal line crossing the low edge of the right scapula, with the most leftward electrode placed at the crossing point between this line and the spine.
As we have discussed before (see article I of this series), sometimes we are unable to determine the baseline lung impedance (BLI), but instead the instantaneous lung impedance (ILI), and in this case, as ILI is largely influenced by individual anthropometric characteristics, it would be better to obtain the variation of lung impedance (ΔLIR).
MONITORIA will use an algorithm that subtracts electrode-skin and under-skin tissue impedance from the total transthoracic impedance, resulting in the net ILI, which renders the device more sensitive to small changes in pulmonary fluid content or congestion and less dependent on individual changes in electrode-skin and under-skin impedance changes.9,10 Then, calculations of lung fluid status according to the equation that states that ΔLIR=[(ILI/BLI)-1]x100%, will provide us PC stands for pulmonary congestionin relative values, making this parameter less dependent on individual patient and device characteristics.
Impedance will be measured at night, every 30 min, from midnight until 6 am, ensuring that the patient is in a supine position, and not performing any physical activity. This way, lung bioimpedance measurements will be more accurate. It may seem strict and inappropriate since patients have to remain quiet all night and some patients have told us that they could not have sexual intercourse for instance, but it must be remembered that this strict schedule is for the sake of accuracy during the validation study of MONITORIA, so some strict rules are necessary. But in real life, when the validated MONITORIA device is used in clinical practice, patients will be free to move during the night, and the interpretation of data will take that into account. The need to go to bed and sleep at a pre-specified time does not seem to be a problem because the device is programmed to take measurements from midnight. The majority of patients will be in bed very before that time and most will be over the age of 80.
The impedance electrodes will also enable the assessment of impedance cardiography for blood pressure (BP) determination, cardiac output and the pre-ejection period.
Electrocardiographic dataThe ECG electrodes used are dry electrodes made from nylon fibers covered with silver. As shown by Taji et al.11 these provide the best interface between skin and the sensors. They are positioned as depicted in Figure 1, which according to Noh et al.12 are the best locations for electrocardiogram acquisition with minimal interference.
We chose to use a set of nine electrodes, including the six standard electrodes for precordial leads (V1, V2, V3, V4, V5 and V6) and three for the limb leads (DI, DII and DIII), because of the need to obtain a more comprehensive electrical perspective of the heart, as we intend to monitor not only heart rate (HR) and heart rate variability (HRV) but also other sensitive variables such as T wave heterogeneity/variability (TWH/TWV), conduction abnormalities and the possible occurrence of any sign of myocardial ischemia. An additional 10th electrode is placed 2 cm below xiphoid appendix, to function as a neutral electrode (see Figure 1).
Electrocardiogram data will be acquired continuously and stored transiently on the patient's mobile phone and then, permanently on the cloud server.
For analyses of HRV we will use the long-term recording of frequency-domain method, in which very low frequency, low frequency and high frequency are calculated from spectral imaging of the ECG recording and analyzed over a 24 h period.
For analyses of TWH/TWV we will use the multi-lead template-derived residua algorithm described previously by Nearing et al.13 as this has been shown to create a baseline median-beat template for each lead and subtracting this template from the beat stream it is then used to determine the morphologic changes that are truly potentially arrhythmogenic, such as R wave heterogeneity (RWH) and TWH.
Right atrial and left atrial pressuresMONITORIA will use near-infrared spectroscopy (NIRS) to assess right atrial pressure (RAP) and left atrial pressure (LAP) and LAP. The location of the sensors will be similar to that used by Pellicori et al.14 but with some adaptations, in order to make the device more comfortable for the patient and not so worrisome.
To measure RAP we used a sensor placed at the 3rd right intercostal space adjacent to the sternum and a zero-reference sensor placed at the 3rd intercostal space in the right mid-axillar line. To measure LAP we used a sensor placed at the 3rd left intercostal space adjacent to the sternum and a zero-reference electrode placed at 3rd intercostal space in the left mid-axillar line (see Figure 1).
Measurements of RAP and LAP will take place (each 30 min) at night, from midnight to 6 am, assuring that the patient is at the supine position and with no or minimal body movement. The explanation of this is the same as described above in section A.
Photoplethysmography sensor systemFor BP measurements, MONITORIA is designed with a photoplethysmography (PPG) sensor located at the xiphoid appendix level (Figure 1) and to use, ECG and bioimpedance data with respective electrodes positioned as already described above. An algorithm is in development to integrate data from all of these three sources (ECG, bioimpedance and PPG) and use them to calculate systolic and diastolic blood pressure, using an adaptation of the Van Lien et al. formula.15 The algorithm will also be able to integrate the accelerometer data not only to identify the most favorable moments for pulse transit time (PTT) measurement (a parameter needed to calculate BP), but also to eliminate noise, breathing movements and other artifacts associated with the raw data captured by the electrocardiographic, bioimpedance and PPG sensors.
Blood pressure is measured continuously and stored transiently in the patient's mobile phone and permanently in a cloud server.
Pre-ejection periodThe pre-ejection period (PEP) is an index that links electrical and mechanical cardiac activity, and is obtained by simultaneous recording of the electrocardiogram and impedance cardiogram. It is defined as the interval between the onset of ventricular depolarization (Q-wave onset in the ECG) and the opening of the semilunar valves, i.e., ventricular contraction (sharp upstroke in the dz/dz or B-point in the impedance cardiogram).
When left ventricular failure occurs, the pre-ejection period lengthens (reduced left ventricular pressure during ventricular contraction) and the left ventricular ejection time shortens. Non-invasive assessment of the pre-ejection period and left ventricular ejection time could assist safe and accurate prescription and monitoring therapies for patients with HF, as we have described above. Even though we know that it is influenced by sympathetic activity and becomes shorter under stimulation, errors can occur in interpretation in the presence of BP increases, which can induce a lengthening of PEP (instead of the expected shortening).
Peripheral oxygen saturation sensor systemThe oximetry sensor block is composed of a concentric arrangement of eight photodiodes around two LEDs, one infrared and one red, as proposed by Fontaine et al.16 The LEDs are 1 cm apart from each other, and the surrounding photodiodes (also 1 cm apart from LEDs and placed at their same level, at contact skin surface) will be able to pick up light from nearly every angle. The LEDs in the sensor module are driven by a frequency of 100 Hz with a 2.5 ms delay between each LED as it is turned on. The circuitry will be arranged so as to allow the block sensor to be embedded into the thoracic vest in a most comfortable way for the patient, ensuring its correct positioning in the thorax, more precisely in the proximal region of the sternum, immediately below the manubriosternal joint.
Once the signals are separated and identified, the AC components of the red and infrared signals are measured using the peak-to-peak method and the DC components are measured by computing their respective averages. As soon as the signals are processed, peripheral oxygen saturation (SpO2) can be calculated as the ratio of the red AC/DC divided by the infrared AC/DC multiplied by -25 and added to 110. In order to provide a SpO2 measurement between 0 and 100%, the SpO2 calculation is then divided by 110 and multiplied by 100 so that the value is normalized.
Oxygen saturation is captured continuously and stored transiently in the patient's mobile phone and permanently in a cloud server.
Skin sodium content measurementsMONITORIA will measure skin sodium using a microfluidic patch (with chemical transducers inside) that absorbs sweat on the surface of the skin. In the validation study, measurements should be taken in a fasting period (we scheduled this first measurement to occur at 0.600) and two hours after each main meal (we scheduled these to occur at 10:30, 15.00 and 22.00).
As we do not know the real normal values of skin sodium in HF patients, nor values predicting HF decompensations because of pulmonary congestion, we will measure it in normal individuals in parallel to sodium in urine and feces (as well as saliva) to determine normal values of sodium excretion through the skin; during this period sodium intake has to be controlled. This will be part of the pilot study, which will, in the meantime, be carried out in order to study the accuracy of the device. Then, some HF patients will be on medication as well as not treated (the patient will be once the treated one and once the control one, with no medication). Data have to be adjusted according to the medication and its dosing, and integrated with other biodata from the each patient.
Berger et al.17 stated that an HF patient with edema excreted 8-13 mEq/day; but in the HF patient described by Haugen, in a report from 1956,18 with HF that was not correctly treated and no edema, it was 26 mEq/day; hence, we speculate that normal excretion may be near 39 mEq/day.
Three-axis gyroscope and accelerometerA three-axis gyroscope will be used to measure lift from the ground and orientation of the body together with a three-axis accelerometer.
The three-axis accelerometer will enable capture of the three main components of acceleration signal (dorso-ventral, head-to-foot and lateral) for measurement of body movements. This will provide us with frequency and duration of the patient's physical activity.
These activity sensors will be used, also, to identify optimal periods for measurements of PPG and its proportional PTT, as well as to suppress motion/respiratory artifacts, in order to provide clearer electrocardiogram, bioimpedance and oximetry data.
The general components of MONITORIA deviceMONITORIA is a home monitoring device, designed for the follow-up of HF patients, and consists of the following main components:
Wearable microcontrollerA wearable microcontroller-based monitoring device imbedded in a thoracic vest designed to be comfortable (made from a soft and specific textile designed to function as a second skin so it is well tolerated by patients, especially by the elderly) for both sexes and body shapes, planned to measure the vital, electrophysiological, hemodynamic and chemical variables described in the previous section. Measurements are taken continuously and transmitted in a raw format to the server on a daily basis. The three axis-accelerometer included in MONITORIA will assist with artifact removal in addition to providing information on posture and the patient's physical activity.
Software applicationA software application, which is meant to run on a patient's smartphone, integrates measurements captured from the wearable device via Bluetooth low energy (BLE) which helps achieve a longer battery life, and forwards them to a cloud server (integrated in an encrypted and secure network), via the cellular network.
Server solutionWe included a server solution for aggregation and assessment of data. This is designed employing an encrypted cloud storage service.
Medical applicationMONITORIA has a PC Java application allowing health personnel to integrate remote monitoring into their workflow. When logging in to the medical application, doctors receive an overview of all patients connected to the system and their overall status. Then, it is possible to study the individual patients and their recent and post monitoring history in two ways: using the option of viewing measurements, which shows the parameters monitored, in a raw data format, or using a statistical view, which shows the trends of the parameters monitored for the last 30 days.
BatteryThe device and uses a lithium polymer batter which must be changed every 24h.
Bluetooth low energy connectionThe device measures data continuously and data are stored locally when there is no BLE connection to the patient's mobile application, thus allowing patients to leave the phone behind when going out of the house, without any data being lost. When the bluetooth low energy (BLE) connection is restored, data is then transferred to the smartphone application, and from there, to the server solution.
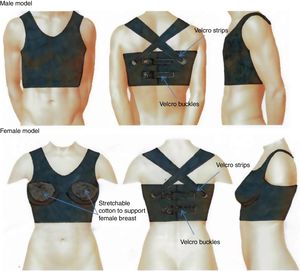
The thoracic vestMONITORIA's electronic sensors and circuitry are embedded in a thoracic vest designed to fit both sexes, and adjustable to different body shapes by means of VelcroTM buckles on the back. This thoracic vest is made of a breathable elastic material which has to be washable and user-friendly. It has two main parts: the thoracic vest itself (washable part) and a flexible platform to be placed inside the thoracic vest, containing all the circuitry components.
The part with the electronic components is a flexible printed circuit board covered in mechanically flexible polyethylene terephthalate (PET) substrate, where the tips of the electrodes, the sensors’ surface and the rechargeable battery are located externally. This part must be fixed to the textile enclosure to avoid relative motion between them.
To ensure water protection in the circuitry region, the textile vest that covers it must be hydrophobic on the inner surface – in contact with the PET substrate containing the circuit – and hydrophilic on the outside – in contact with the skin. Gore-Tex19 is an example of this kind of material, as it is breathable, stretchable and has a double layered membrane with hydrophobic and hydrophilic surfaces. To improve the patient's comfort, the remaining area of the vest, which does not contain any circuitry inside, has to be made from a washable and stretchy type of cotton.20 The hydrophilic side (in contact with the skin) must have holes with the shape and size of the electrodes and sensor's surface (to allow direct contact between the sensors with the skin), and the area surrounding these holes should be made of a silicone gel, in order to improve the steadiness of the electrodes/sensors in contact with the skin.
Finally, to guarantee that the thoracic vest is worn properly and adjusted to the patient's thorax we decided to include a tight-switch sensor in the vest.
Figure 2 shows the MONITORIA thoracic vest.
Software design- -
Patient mobile phone application:
All the data given by the sensors are recorded transiently in the patient's mobile phone, before being sent to the cloud server. The patient's mobile phone will have an application, specifically developed for MONITORIA, which instructs the patients whenever appropriate. This will inform them of the most suitable time for meals (ensuring the correct measurement of skin sodium content) and giving them the feedback regarding their physical activity, informing them if they have already reached the average physical activity required for each, or advising them to walk a little more. This patient feedback will be displayed on the phone screen in written form, but sound commands will also be issued ensuring that those who are unable to read or write and/or visually impaired patients can also understand the instructions provided.
Moreover, as part of their daily hygiene, patients must remove the thoracic vest when they bathe, but this period should never exceed 30 minutes. Whenever this happens the phone app will emit an alarm informing the patients that they have to put the thoracic vest back on again.
- -
Medical application:
The patient's mobile phone application uploads the data to the cloud-based server every 24h to make this information available to the attending physician.
Then, according to a pre-specified protocol, the physician will access a passworded medical application on their personal computer, specifically developed for MONITORIA, retrieving all the data recorded in the cloud.
Once the application is open, the default page gives an overview of all the patients connected to the system. Then the physician can choose which patient to analyze in more detail. The individual patient's page shows, by default, the summary statistics of each parameter for the last 24 h, 7 days or 30 days (detailed statistics visualized by clicking on each icon). There is also the possibility of changing this view to a raw format data, assessing all data without any statistical processing.
The medical application also provides an alarm menu which displays all the alarms emitted in the last 30 days, specifying their nature, the professional contacted (if it was only the attending physician or also the national emergency system) and the success of the actions taken.
Operating mode and alarm systemThe MONITORIA sensors will be activated when the healthcare provider dresses the patient, for the first time, in the thoracic vest. At this time the physician will, also, determine if the system needs the cardioverter-defibrillator function as well (with an upgraded version, see below).
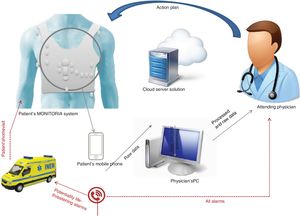
The MONITORIA performs continuous real time capture, analysis and transmission of all data on a daily basis. Significant alterations in a patient's variables (see alarms below) alert the attending physician immediately via a text message. This message includes the name of the patient, their telephone number and address, and information concerning the alarm (mainly nature and duration of the event).
Whenever the physician receives an alert, they immediately make a phone call to the patient to gauge their clinical status, and depending on the nature of the alarm and the information directly provided by the patient or their relatives, the physician will instruct the patient on what to do next. This action plan may include alteration in therapeutics (adding/stopping medication, altering dose) or scheduling an urgent appointment at a day hospital/outpatient clinic. Figure 3 presents an overview of MONITORIA's remote monitoring system.
Whenever there is a potentially life-threatening or lethal situation (imminent or consummate cardiac arrest, potential myocardial infarction…), the system will also send an alert to the national emergency medical system and a medical emergency team will be sent immediately to the patient's home. In order to improve the specificity and sensitivity of this function the basal ECG of each patient must be acquired and registered previously and only new abnormalities considered. This personalized methodology is necessary to avoid pitfalls in diagnosis.
In the future, MONITORIA will be able to send shocks or function as a pacemaker to treat certain arrhythmias (ventricular fibrillation [>30 sec and HR≥200 bpm] or sustained ventricular tachycardia [>30 sec] with HR of ≥150 bpm) or blockade/pause >3 sec, as suitable. We are now studying the best specifications to avoid inappropriate shocks as this is associated with high mortality and morbidity.
The decision to activate the cardioverter defibrillator function would be taken by the physician the very first time the patient utilizes the device. If the patient has ischemic heart disease or a cardiomyopathy with reduced left ventricular ejection fraction (special caution is required when ≤35%), the cardioverter defibrillator function should be activated. This is because Röger et al.21 found that patients with LVEF>35% did not benefit from cardioverter defibrillator. In MONITORIA the first shock will be 150 J. If the arrhythmia occurs at separate episodes, with ≥3 min in between, then the system will consider them separate arrhythmias and will fire separate shocks.
MONITORIA generates alarms in the following situations:
- -
New onset of atrial fibrillation, atrial fibrillation with rapid ventricular rate, sustained ventricular tachycardia, ventricular fibrillation;
- -
Electric conduction pauses ≥2 secs or complete atrioventricular block. De novo right bundle branch block;
- -
A 2 mm rise in ST segment in two out of three leads in each group (limb or precordial leads) generating high suspicion of myocardial ischemia;
- -
Heart rate ≤40bpm or ≥120bpm, lasting more than 5 min, and no (or minimal) physical activity detected;
- -
Peripheral oxygen saturation ≤89%, lasting more than 5 min;
- -
Instantaneous lung impedance ≤100 Ω, in three consecutive measurements, or ΔLIR<18%;
- -
Systolic blood pressure ≤70 mmHg or ≥200 mmHg; and/or diastolic blood pressure ≤35 mmHg or ≥100 mmHg, lasting at least 5 min;
- -
Right atrial pressure ≥8 mmHg in three consecutive measurements;
- -
Left atrial pressure ≥12 mmHg in three consecutive measurements.
At present, despite technological and pharmacological innovations, HF continues to cause a huge public health impact and is a major economic and social burden.
In this paper we present MONITORIA, a promising device that we believe will change the paradigm of ambulatory follow-up of patients with HF, essentially because it is part of a follow-up program (the GENICA project) that takes into account a multi-perspective feature of HF development. It is based on real world patients, adapting innovations not to the disease but rather to patients.
In the near future, predictive analysis will be built upon this technology by establishing algorithms for preventing clinical events such as impending HF decompensation before it occurs in the outpatient setting.
As soon as the MONITORIA's development and prototype testing phase is concluded, an article (part III of this series of three articles) will be published reporting the results of validation tests.
MONITORIA and its follow-up strategy will provide optimization of HF patient care, reducing significantly their exacerbations, hospital admissions (hospitalizations) and mortality, thus reducing costs. Consequently, HF-associated burden will also be attacked at the source.
LimitationsMONITORIA has some limitations, mainly related to the strict schedule; it has to be used continuously so the measurements are taken properly, the patient has to rest during the night so certain measurements are taken accurately and the batteries must be changed every 24h (some patients may not remember this, so for ease we added an alarm as a reminder for patients). This whole system may be complex in patients’ daily lives. However, these limitations may be overcome by the beneficial effect that the accurate monitoring of such a deadly disease can have on patients
A home monitoring programme using MONITORIA may be useful (feasible) after validation for particular subsets of HF patients, especially those with severe forms of HF at higher risk of decompensation and (re)hospitalization, with no cognitive dysfunction or depression, and additionally, those at ease with new technologies or who live with a caregiver who is.
We are now studying the best specifications to avoid inappropriate shocks as this is associated with high mortality and morbidity. This is why this function should only be activated by the responsible physician who is able decide on its use according to patient need to avoid lethal consequences.
FundingThe authors declare that no funding was provided conducting this study or preparation of this manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.