To report medium- and long-term results following a single second-generation cryoballoon (CB2)-based ablation procedure in patients with paroxysmal (PAF) and persistent (PeAF) atrial fibrillation.
MethodsA retrospective study was performed of consecutive patients undergoing a first CB2-based ablation procedure in a tertiary center. Cryoenergy was applied for 3 min if a time to effect <60 s was documented or 4 min otherwise, with a bonus application in cases of late isolation or suboptimal temperature. Follow-up was obtained from the regional health electronic records system and by telephone or personal interviews. Recurrence was defined as any atrial arrhythmia >30 s beyond a three-month blanking period. The clinical impact of recurrences was classified using a severity score.
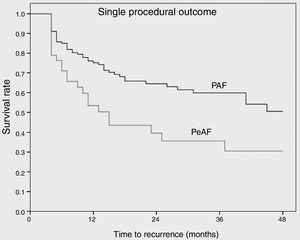
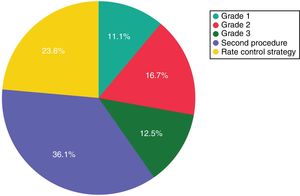
ResultsA total of 172 patients (134 PAF and 38 PeAF) were included, of whom 25 (14.5%) had structural heart disease and 120 (69.7%) had a normal or mildly dilated left atrium. Acute success was achieved in 167 (97.1%). After a median follow-up of 27 (14-41) months, 100 patients (58.1%) remained free of atrial arrhythmias (64.2% for PAF and 36.8% for PeAF, p=0.006). Left atrial size (p=0.05) and clinical presentation as PeAF (p=0.006) were predictors of recurrence. Of patients with recurrences, 11.1% did not require further therapies and an additional 16.7% had good control with antiarrhythmic drugs.
ConclusionsA single CB2 procedure resulted in 58.1% of patients remaining free of atrial arrhythmias at 27-month follow-up. Conservative management was useful in 27.8% of patients with recurrences.
Relatar os resultados a médio e longo prazo após um único procedimento de crioablação com um sistema de segunda geração (CB2) em doentes com fibrilhação auricular paroxística (PAF) e persistente (pAF).
MétodosEstudaram-se retrospetivamente doentes consecutivos referidos para uma primeira ablação com CB2 num centro terciário. A ablação foi efetuada com crioenergia aplicada durante 3 minutos se o tempo do efeito foi < 60 s, ou então foi aplicada durante 4 mim, com uma aplicação extra em caso de isolamento tardio ou temperatura subótima. Realizou-se o acompanhamento por meio do registo do sistema de saúde eletrónico regional e pelo telefone ou com entrevistas pessoais. A recorrência foi definida como qualquer arritmia auricular > 30 segundos depois dum período de três meses. O impacto clínico das recorrências foi classificado utilizando uma pontuação de gravidade.
ResultadosForam estudados 172 doentes (134 PAF e 38 pAF). Vinte cinco (14,5%) tinham doença cardíaca estrutural e 120 (69,7%) tinham uma aurícula esquerda (LA) normal ou levemente dilatada (LA). Foi obtido sucesso agudo em 167 (97,1%). Após um seguimento médio de 27 [14-41] meses, 100 doentes (58,1%) permaneceram livres de arritmias auriculares (64,2% para PAF e 36,8% para pAF, p = 0,006). O tamanho da LA (p = 0,05) e a apresentação clínica de pAF (p = 0,006) foram preditores das recorrências. Dos doentes com recorrências 11,1% não precisaram de terapias adicionais e 16,7% foram bem controlados com medicamentos antiarrítmicos.
ConclusõesDos doentes, 58% ficaram livres de arritmias após um único procedimento CB2, até 27 meses de acompanhamento. Uma estratégia conservadora foi útil nos 27,8% dos doentes com recorrências.
Cryoballoon isolation of the pulmonary veins (PVs) is an alternative approach to radiofrequency ablation (RF) in symptomatic atrial fibrillation (AF). Similar success rates at six and 12 months post-procedure have been achieved with both techniques in several studies.1–3 Since 2012 an improved, second-generation cryoballoon (CB2) (Arctic Front Advance, Medtronic Inc., Minneapolis, MN, USA) with more extensive and effective cooling at the frontal surface of the balloon has become available. The improved cooling characteristics may result in more homogeneous ablation lines, which translate into a higher rate of durable PV isolation (PVI).4,5 Greater freedom from atrial arrhythmias after a single procedure at one-year follow-up has been reported for CB2 compared with non-force sensing conventional RF ablation6 or with first-generation cryoballoons.7 However, few data are available on clinical outcomes beyond one year after a CB2 ablation procedure. The aim of this study is to report medium- and long-term results following a single CB2-based PVI procedure in patients with paroxysmal (PAF) and persistent (PeAF) atrial fibrillation.
MethodsStudy populationAll patients undergoing a first ablation procedure for PAF or PeAF using the CB2 technique in a single tertiary center between June 2012 and June 2017 were retrospectively analyzed. Demographic and clinical data were obtained from clinical records at the hospital where patients were admitted for the index procedure. AF was defined as persistent if episodes lasted >7 days or required cardioversion after >48 h from onset. Long-standing persistent (>1 year) AF patients were not considered for ablation. The study was approved by the local ethics committee.
Periprocedural managementComputed tomography or magnetic resonance angiography was performed before the procedure to assess the morphology of the left atrium and PVs, but a CB2-based PVI procedure was attempted in all patients irrespective of anatomic characteristics. A 28-mm balloon was chosen unless all PVs had a diameter <20 mm, in which case a 23-mm balloon was used. The left atrium was categorized as normal or mildly, moderately or severely dilated according to the American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines.8 Transesophageal echocardiography was performed prior to ablation in all patients to rule out intracardiac thrombi. The procedures were performed under deep sedation or general anesthesia. A single transseptal puncture was performed under fluoroscopic guidance using the Brockenbrough technique and a specially designed 12-F (15-F external) steerable sheath (FlexCath Advance, Medtronic) was introduced into the left atrium. An initial bolus followed by perfusion of heparin was administered targeting an activated clotting time of 300-350 s. The CB2 was advanced towards the ostium of each PV over a spiral mapping catheter (20-mm Achieve™, Medtronic), which was manipulated to assess the PV electrograms. The CB2 was inflated and pushed against the PV ostium to completely occlude the antral aspect of the vein. Complete PV antrum occlusion was verified by radiocontrast injection. If the time to effect, as assessed by the disappearance of the PV potentials in the spiral catheter, was less than 60 s, cryoenergy application was continued to complete a 3-min cycle, otherwise a 4-min cycle was delivered. A bonus application was given in cases of late isolation of the vein or minimum achieved temperature >-40°C. During ablation of the right PVs continuous phrenic nerve pacing was performed from the superior vena cava to prevent phrenic nerve palsy. If PV isolation could not be achieved after several CB2 attempts, touch-up ablation using RF energy was applied to the residual gaps in order to complete the block line.
Follow-upClinical follow-up was obtained in all except two patients, who were excluded from the analysis. Data were obtained from the reports of outpatient clinic and emergency department visits and hospital admissions, all available in the electronic health records system shared by all the public health services in the area. Telephone or personal interviews were performed if the available information was not clear or complete. The follow-up strategy was not uniform for all the referral centers, but generally included outpatient visits every 3-6 months, one or two 24-h Holter monitoring periods, and intensive surveillance with repeated and/or prolonged monitoring in patients with potentially arrhythmic, non-documented symptoms. Recurrence was defined as any electrocardiographically documented episode of AF, atrial flutter or atrial tachycardia lasting more than 30 s and occurring beyond a three-month blanking period. Recurrences were classified using a clinical severity score as grade 1: isolated/mild symptomatic recurrences not requiring antiarrhythmic treatment, grade 2: recurrences with good symptom control by an antiarrhythmic drug, and grade 3: symptomatic recurrences despite pharmacological treatment.
Statistical analysisContinuous variables are shown as mean ± standard deviation if normally distributed or as median (interquartile range) otherwise. The effect of discrete variables on arrhythmia recurrence was studied using Kaplan-Meier survival analysis with the log-rank test. Parameters significantly associated with outcome in univariate analysis were included in the multivariate Cox regression model using the step-down procedure. All p-values are two-sided and considered significant if p<0.05. All statistical calculations were performed with IBM SPSS software (IBM SPSS Statistics for Windows, Version 17.0, IBM SPSS Inc.).
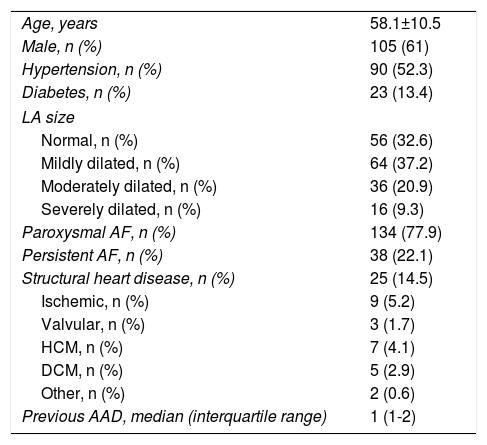
ResultsA total of 172 patients (105 male [61%], age 58.1±10.5 years) were included in the study. PAF was present in 134 (77.9%) and PeAF in 38 (22.1%). Twenty-five patients (14.5%) had structural heart disease (nine ischemic, seven hypertrophic cardiomyopathy, five dilated cardiomyopathy). The left atrium was of normal size in 56 patients (32.6%), and was mildly, moderately or severely dilated in 64 (37.2%), 36 (20.9%) and 16 (9.3%), respectively. Other baseline characteristics are shown in Table 1.
Baseline characteristics of the patient population (n=172).
| Age, years | 58.1±10.5 |
| Male, n (%) | 105 (61) |
| Hypertension, n (%) | 90 (52.3) |
| Diabetes, n (%) | 23 (13.4) |
| LA size | |
| Normal, n (%) | 56 (32.6) |
| Mildly dilated, n (%) | 64 (37.2) |
| Moderately dilated, n (%) | 36 (20.9) |
| Severely dilated, n (%) | 16 (9.3) |
| Paroxysmal AF, n (%) | 134 (77.9) |
| Persistent AF, n (%) | 38 (22.1) |
| Structural heart disease, n (%) | 25 (14.5) |
| Ischemic, n (%) | 9 (5.2) |
| Valvular, n (%) | 3 (1.7) |
| HCM, n (%) | 7 (4.1) |
| DCM, n (%) | 5 (2.9) |
| Other, n (%) | 2 (0.6) |
| Previous AAD, median (interquartile range) | 1 (1-2) |
AAD: antiarrhythmic drugs; AF: atrial fibrillation; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LA: left atrial.
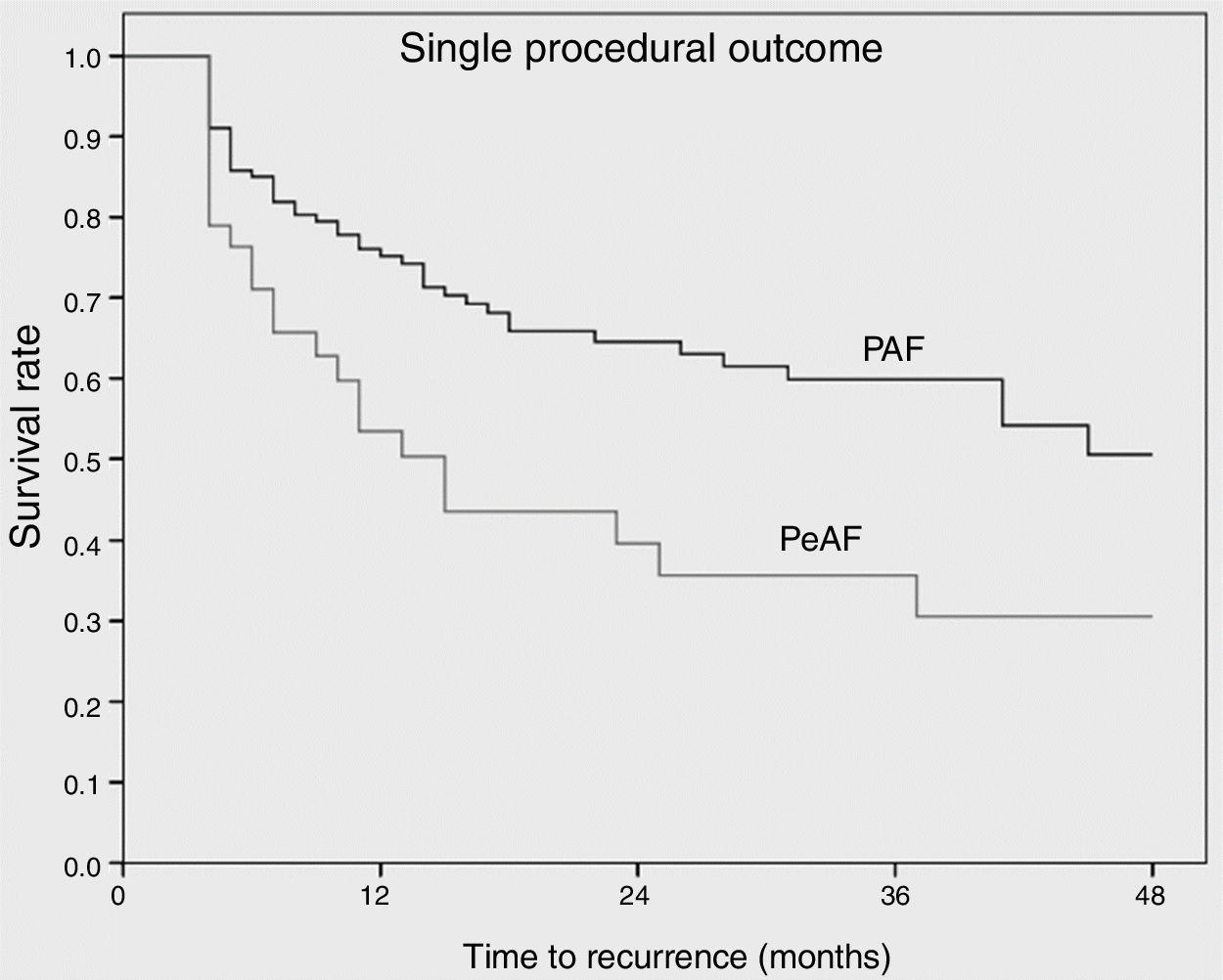
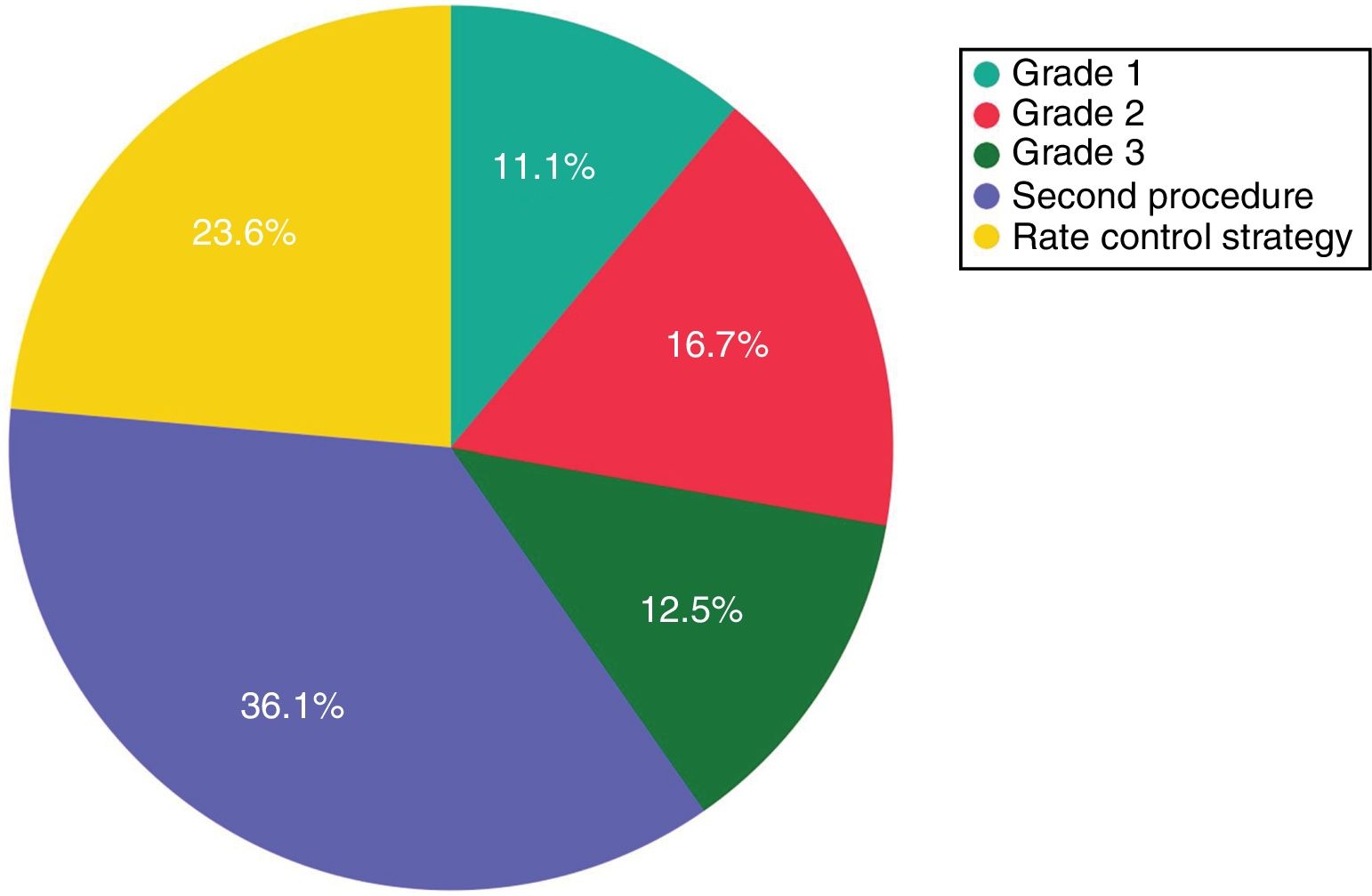
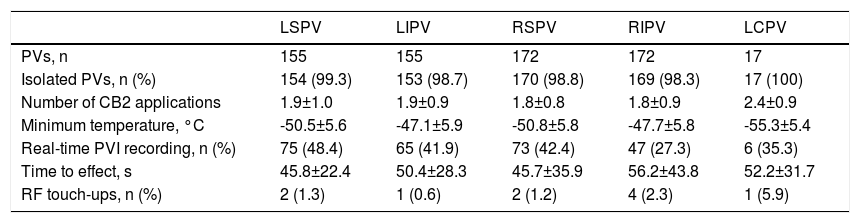
Complete bidirectional isolation of all PVs was achieved in 167 patients (97.1%). Procedure time was 164±57 min and the radiation dose-area product was 21.7 (8-27) μGy.m2. A 23-mm balloon was used in four patients (2.3%). Detailed periprocedural data are shown in Table 2. Five patients (2.9%) presented local vascular complications and one patient with an apparently uneventful PVI procedure using a 28-mm balloon and with no need for RF application developed symptomatic PV stenosis several months after the procedure, requiring PV stenting. No cases of persistent phrenic nerve palsy, stroke/transient ischemic attack or atrioesophageal fistula were observed. After a median follow-up of 27 (14-41) months, including the three-month blanking period, 100 patients (58.1%) remained free of atrial arrhythmias with a single cryoballoon procedure. The rate of recurrence was 35.8% for PAF and 63.2% for PeAF patients (odds ratio: 3.1, p=0.006) (Figure 1). Left atrial size (p=0.05) and clinical presentation of AF as persistent (p=0.006) were significantly related to arrhythmia recurrence after the blanking period, but only the presence of PeAF remained a significant predictor in multivariate analysis. The clinical severity of symptoms and the final outcome of patients with recurrences are presented in Figure 2. Patients with a severity score <3 remained in good clinical condition with conservative management at 24 (8-43) months after the first documented recurrence. The rate of patients with substantial clinical improvement, defined as absence of recurrence or recurrence with severity score 1 after a single cryoballoon procedure, was 73% at one year (77% in PAF vs. 56% in PeAF), 63% at two years (69 vs. 44) and 60% after three years (64 vs. 44), respectively.
Procedural characteristics.
| LSPV | LIPV | RSPV | RIPV | LCPV | |
|---|---|---|---|---|---|
| PVs, n | 155 | 155 | 172 | 172 | 17 |
| Isolated PVs, n (%) | 154 (99.3) | 153 (98.7) | 170 (98.8) | 169 (98.3) | 17 (100) |
| Number of CB2 applications | 1.9±1.0 | 1.9±0.9 | 1.8±0.8 | 1.8±0.9 | 2.4±0.9 |
| Minimum temperature, °C | -50.5±5.6 | -47.1±5.9 | -50.8±5.8 | -47.7±5.8 | -55.3±5.4 |
| Real-time PVI recording, n (%) | 75 (48.4) | 65 (41.9) | 73 (42.4) | 47 (27.3) | 6 (35.3) |
| Time to effect, s | 45.8±22.4 | 50.4±28.3 | 45.7±35.9 | 56.2±43.8 | 52.2±31.7 |
| RF touch-ups, n (%) | 2 (1.3) | 1 (0.6) | 2 (1.2) | 4 (2.3) | 1 (5.9) |
Variables are presented as mean (SD) or n (%), as appropriate.
LCPV: left common pulmonary vein; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; PV: pulmonary vein; PVI: pulmonary vein isolation; RIPV: right inferior pulmonary vein; RSPV: right superior pulmonary vein; RF: radiofrequency.
Clinical severity and final outcome of patients with recurrences after a single cryoballoon procedure. Grade 1: isolated/mild symptomatic recurrences not requiring antiarrhythmic treatment; grade 2: recurrences with good symptom control by an antiarrhythmic drug; grade 3: symptomatic recurrences despite pharmacological treatment.
Few and sometimes conflicting data have been reported on long-term clinical outcomes in patients with PAF and PeAF undergoing CB2-based PVI. In our series the survival rate free of arrhythmias was 75% and 60% at one and three years, respectively, for patients with PAF. However, for PeAF patients the corresponding figures were significantly lower, at 50% and 31%. The clinical severity of recurrences is not usually addressed in the literature, but this parameter may be important from a clinical standpoint. Our results suggest that approximately one fourth of patients with recurrence may do well with a conservative strategy.
In patients with PAF, two recent studies compared the mid-term results of CB2 versus contact-force sensing RF. Both included a small number of patients treated with CB2 and reported success rates of 75-80% at one year and 67-72% at two years of follow-up.9,10 Our results are similar to these studies and slightly inferior to the 85-90% patients free of arrhythmia at one year reported in another two series of patients with PAF.11,12 The reason for this better outcome is not clear, since the population characteristics and the follow-up procedures based on assessment of symptoms and additional non-invasive Holter recordings were similar. Regarding patients with PeAF, very different arrhythmia-free survival rates at 2-3 years of follow-up after a single CB2 procedure have been reported, ranging from 39%11 to more than 70%.12,13 Our results are in agreement with the lower figures, suggesting that a single CB2 procedure has limited success for the long-term control of PeAF. The large differences may be related more to difficulty in the classification of the arrhythmia as PAF or PeAF, which can be difficult in many patients, than to the intrinsic characteristics of the procedure. Although a bonus freeze and an additional roof line were used in several patients in the series with the best results, the authors did not observe significant differences in this regard.
Data on the clinical severity of recurrences and the ability to control them using antiarrhythmic drugs that were ineffective before the CB2 procedure are lacking in the literature. In a recent study the recurrence rate at one-year follow-up after a single cryoballoon PVI in patients with PeAF was 39.3%, but only 16% had arrhythmia-related symptoms after the ablation procedure. Moreover, a significant and clinically meaningful improvement in several quality-of-life scores was also observed in the vast majority of patients, suggesting to the authors that some procedures might be considered a clinical success despite a documented arrhythmia recurrence.14 In our series, with a longer follow-up period, most patients with clinically documented recurrence will need antiarrhythmic drugs, a second procedure or a switch to a rate control strategy. However, approximately 11% of patients with recurrences will have a good medium-term clinical course and continue to have a low burden of symptomatic arrhythmias, not requiring further use of pharmacological or invasive therapies to control the episodes. This is a small but not negligible percentage and may support a conservative strategy after a first isolated late recurrence, even if it has been symptomatic. The percentage of patients with clinically significant improvement increases if those who have good control of the arrhythmia under antiarrhythmic drugs that were not effective before the ablation procedure are included, since according to our results 16.7% of patients with recurrences may have a good clinical response to this approach.
Study limitationsFirst, our findings represent the experience of a single medium-volume center performing 50-70 AF ablation procedures per year over the last 10 years, most of them using the cryoballoon technique. Although this number is relatively low, our high rate of successful acute PVI and our low complication rates suggest that this volume is sufficient to maintain the skills required for the procedure. Second, the study is retrospective and the clinical management and follow-up schedule after the procedure was at the discretion of the referring cardiologist. However, all patients had an outpatient visit every 6-12 months and several 12-lead electrocardiograms and Holter recordings were performed if the patient had symptoms potentially indicative of an arrhythmic origin.
ConclusionsAt a median follow-up of 27 months, 58.1% of patients remained free of atrial arrhythmias after a single CB2 procedure. The success rate was significantly higher for PAF. No further therapy was needed in 11.1% of patients with recurrences and an additional 16.7% had good symptomatic control with antiarrhythmic drugs.
Conflicts of interestThe authors’ institution has received research and training grants from Medtronic.
We thank Rebeca Goya and Oscar Barquero for the translation into Portuguese of parts of this manuscript.