Lipoprotein(a) [Lp(a)] is an independent cardiovascular risk factor but is closely associated with other similar risk factors that are manageable with appropriate treatment and guidance. We aimed to study the impact of using combined therapy for managing Lp(a) levels in patients at high cardiovascular risk but without major adverse cardiovascular events, in primary prevention.
MethodsWe conducted a retrospective observational study in 516 patients randomly selected from a group of 1677 patients who attended cardiovascular risk and metabolism consultations between 1995 and 2015. The disorders observed and therapies used were classified into nosological and pharmacological groups, respectively. Cardiovascular risk was calculated based on the Framingham risk score, the European Society of Cardiology's SCORE and the American College of Cardiology's ASCVD Risk Estimator, and changes in patients’ lifestyle were assessed.
ResultsSignificant differences (p<0.001) were found in almost all metabolic variables, except fasting insulin and C-peptide. Lp(a) levels were also significantly reduced (p<0.001). Carotid intima-media thickness improved, decreasing from 2.90 mm to 1.40 mm; however, there was no reduction in the number of cases of vascular stenosis. Of patients with hepatic steatosis (85.5%), 40.7% presented hepatomegaly, but liver function was only altered in a few patients (14.5%). Lipid-lowering therapy, especially statins, significantly decreased Lp(a), benefiting from synergy with other treatments.
ConclusionsLp(a) is a key overall indicator of vascular risk and should be considered a therapeutic target. Besides a healthy lifestyle, primary prevention should include combined drug therapies to address all cardiovascular risk factors and to delay the atherosclerotic process.
Lp(a) é um fator de risco cardiovascular independente, mas intrinsecamente associado a outros fatores de risco similares, controláveis com terapêuticas e orientações adequadas. O nosso objetivo é estudar o impacto do uso de terapêuticas combinadas na gestão da evolução da Lp(a) em doentes com elevado risco vascular, sem ECVM em prevenção primária.
MétodosEstudo observacional retrospetivo realizado em 516 doentes, selecionados aleatoriamente de um universo de 1677 indivíduos, que participaram regularmente em consultas de risco vascular e metabolismo entre 1995 e 2015. As patologias observadas e terapêuticas utilizadas foram distribuídas em diferentes grupos nosológicos e grupos farmacológicos, respetivamente. Calculou-se o RV com base em FRS, SCORE e ASCVD e avaliou-se também a evolução do estilo de vida dos doentes.
ResultadosEncontraram-se diferenças significativas (p<0,001) em quase todas as variáveis metabólicas, exceto insulina (jejum) e péptido-C. Houve uma redução significativa nos níveis de Lp(a) (p<0,001). A espessura íntima-média carotídea evoluiu favoravelmente, diminuindo de 2,90 mm para 1,40 mm; porém, não houve redução do número de casos de estenose vascular. Dos doentes com esteatose hepática (85,5%), 40,7% apresentaram hepatomegalia. Contudo, poucos doentes (14,5%) apresentaram função hepática alterada. A terapêutica antidislipidémica, especialmente as estatinas, diminuiu significativamente a Lp(a), beneficiando da sinergia com demais tratamentos.
ConclusõesLp(a) é um indicador global e fundamental de risco vascular, a considerar como alvo terapêutico. Além de um estilo de vida saudável, a prevenção primária deve incluir terapêuticas farmacológicas combinadas dirigidas aos fatores de risco cardiovasculares e, consequentemente, retardar o processo aterosclerótico.
angiotensin-converting enzyme inhibitor
ambulatory blood pressure monitoring
angiotensin receptor blocker
apolipoprotein A
body mass index
coronary artery disease
calcium channel blocker
C-reactive protein
cardiovascular disease
Framingham risk score
high-density lipoprotein
carotid intima-media thickness
lipoprotein(a)
low-density lipoprotein
major acute cardiovascular events
non-alcoholic fatty liver disease
oral antidiabetic
peripheral arterial disease
Systematic Coronary Risk Evaluation
selective serotonin reuptake inhibitors
total cholesterol
Lipoprotein(a) [Lp(a)] is identical to low-density lipoprotein (LDL) except for the addition of apolipoprotein A (apoA), which is highly glycosylated. There is a striking homology between the amino acid sequences of apoA and plasminogen, which is recognized to be a cardiovascular risk factor.1 Thus, Lp(a) may play an important role in the transition from atherosclerosis to thrombosis, because it activates monocyte adhesion and migration of macrophage foam cells into the arterial wall.2 Lp(a) is often considered a marker of thrombosis.3
Cardiovascular disease (CVD) is a major cause of death in patients with peripheral arterial disease (PAD). These patients also tend to suffer from complications when they have diabetes, dyslipidemia and hypertension. They may also develop severe systemic atherosclerosis, leading to increased mortality due to coronary artery disease (CAD).
High Lp(a) is positively associated with coronary artery calcification, CAD and PAD.4,5 It also promotes thrombosis by binding to fibrin, thus blocking the fibrinolytic action of plasmin.2 Lp(a) may be a predictor of peripheral and central CVD in younger men and women with dyslipidemia.
Several observations suggest that targeting Lp(a) could decrease total residual cardiovascular risk, as increased plasma Lp(a) concentrations are significantly associated with higher risk of CAD.6
Lp(a) is a marker of particular risk for poor outcomes in terms of severity and progression of CVD. Several prospective studies have correlated Lp(a) levels with vascular disease in general, and plasma Lp(a) >30 mg/dl with increased cardiovascular risk.4
Studies with statins alone reveal that, although they significantly reduce LDL and major adverse cardiovascular events (MACE), statins do not appear to reduce Lp(a) concentrations. Almost all studies in this field have targeted total cholesterol (TC) and LDL, as well as high-density lipoprotein cholesterol (HDL), but none has validated the use of HDL as a therapeutic target in the management of cardiovascular risk factors. Furthermore, in clinical practice cardiovascular risk scores are not usually applied to younger patients (aged <40 years) with lower HDL levels, even if they have other cardiovascular risk factors. We therefore aimed to assess the possible relevance of all lipid fractions, including HDL, to cardiovascular risk factors, regardless of age, and following current clinical guidelines.7–9 In this study, we assessed clinical and biochemical changes in randomly selected patients with two or more cardiovascular risk factors, in primary prevention, with no known cardiovascular events, and considering their social, cultural and demographic characteristics, before and after the patients underwent treatment and medical guidance, and the impact on metabolism and lipid, C-reactive protein (CRP), fibrinogen and homocysteine levels, among other clinical parameters, including Lp(a) profile according to the criteria of the BiomarCaRE consortium.10
MethodsThis retrospective observational study was conducted in 516 patients randomly selected from a group of 1677 patients who attended cardiovascular risk and metabolism consultations between 1995 and 2015, in primary prevention, and had not suffered MACE. The selection criteria were as follows: at least two personal and/or family cardiovascular risk factors, regular attendance at a three-monthly consultation for a minimum of two years, and an annual biochemical assessment, including cardiovascular exams and clinical assessment. Participants filled in a self-administered questionnaire and provided written informed consent for inclusion in the study, which was approved by the Ethics Committee of São João Hospital Center, Porto. Patients were followed for both clinical and anthropometric changes during the observation period, paying particular attention to lipid and metabolic profiles and variations in Lp(a) levels. The disorders diagnosed were classified into the following nosological groups: cardiovascular, cerebrovascular, metabolic and behavioral diseases. Data were collected from the first medical consultation until the last or current appointment recorded on the patient's medical chart. It was established that an individual patient's record could only be updated for specific reasons, such as CVD, dependency or immobility, MACE or death. Records were updated 19 times due to death and six times due to the patient being bedridden.
Patients’ sociodemographic characteristics were analyzed based on their clinical records. Their clinical, anthropometric, biochemical and cardiovascular characteristics were also analyzed and recorded. These assessments included cardiovascular exams, including electrocardiogram (ECG), two-dimensional echocardiogram and Doppler ultrasound (7.5 mHz linear probe, Sonos 1000, Hewlett Packard, Andover, MA) of the supra-aortic trunks to measure carotid intima-media thickness (IMT), as well as ambulatory blood pressure monitoring (ABPM).
Assessment of both renal and liver function are essential to analysis of metabolic status and the effects of pharmaceutical therapy. Renal function was therefore assessed in this study, based on microalbuminuria and creatinine clearance estimated by the Cockcroft-Gault method, and liver function and morphology were assessed by ultrasound.
Cardiovascular risk was calculated based on the three most commonly used scores: the Framingham risk score (FRS), the European Society of Cardiology's Systematic Coronary Risk Evaluation (SCORE), and the American College of Cardiology's atherosclerotic cardiovascular disease risk estimator (ASCVD). The resulting overall scores were analyzed by age-group. Also, due to the association between metabolic compensation and clinical improvement in patients with cardiovascular risk factors, the study included assessment of patients’ lifestyle behaviors, including alcohol consumption (classified as never, occasional, moderate [one or two drinks daily], excessive [three or more drinks daily], alcoholic, or abstinent [at least one year since the last drink]), smoking (current smoker, ex-smoker or never-smoker), and exercise levels (all aerobic exercise at least twice a week in addition to that arising from daily activities).
Finally, drug therapies administered during the observation period were classified into pharmacological groups.
Statistical analysisQuantitative variables were summarized using descriptive statistics: mean, median, standard deviation and range (minimum and maximum). Categorical variables were expressed as absolute (n) and relative (%) frequencies. Associations between two categorical variables were tested using the chi-square test or Fisher's exact test. The McNemar test for paired samples was used to compare the presence of a symptom before and after treatment or to compare the results of two diagnostic tests applied in the same group. Quantitative variables in two independent groups were compared using the t test for independent samples or the Mann-Whitney (MW) non-parametric test, according to whether the respective assumptions were validated. The non-parametric Wilcoxon signed-rank (WSR) test and sign test (ST) were used for paired samples to compare clinical scores before and after treatment if the assumption of normality was not confirmed. Statistical tests were two-tailed and the significance level was taken to be 5%. Multivariate linear regression analysis was performed to assess associations between variables of interest and changes in Lp(a) levels between the beginning and the end of the study period. Stepwise optimization was used to choose the statistically significant variables for the model. All statistical analyses were conducted using IBM® SPSS® Statistics version 19.
ResultsSociodemographic, clinical and anthropometric characteristics of the study populationOf the 516 patients, 224 were male and 292 were female, and 98.6% were Caucasian. The mean observation time was 11.35±4.32 years (range: 2-26), median 11.0 years.
During the observation period, patients’ age increased from an initial median of 46 years to a final median of 58 years. Thus, age grouping was readjusted, as the number of patients decreased drastically in the <20 years (initial: 2.2%; final: 0.2%) and 20-34 years (initial: 20.2%; final: 7.0%) age groups, changed slightly in the 34-50 years age group (initial: 34.3%; final: 22.9%), and increased in the 50-64 years (initial: 27.9%; final: 35.5%) and ≥65 years (initial: 14.9%; final: 34.5%) age groups. By the end of the observation period, 23 patients had died (4.5%) and 41 patients were bedridden (7.9%); most of these events occurred after data collection.
Changes in patients’ clinical and anthropometric assessments between initial and final observations are detailed in Table 1. Significant reductions were seen in waist circumference, upper arm circumference and triceps skinfold (p<0.001). In addition, there were considerable improvements in blood pressure control, reflected in statistically significant differences in mean systolic and diastolic daytime and night-time blood pressure (p<0.001). The number of patients with controlled atrial fibrillation rose from nine to 18 patients during the study period, while the number with non-controlled atrial fibrillation decreased from two to one. No statistical differences were found regarding weight, height or body mass index.
Changes in anthropometric and clinical assessments between initial and final visits.
| n | Mean difference | Median difference | Range (min-max) | p | |
|---|---|---|---|---|---|
| Weight, kg | 516 | -0.54±9.10 | -0.4500 | -40.00-57.60 | 0.069 (WSR) |
| Height, cm | 516 | -0.37±4.73 | 0 | -6.00-86.00 | 0.700 (ST) |
| BMI, kg/m2 | 516 | -0.36±3.23 | -0.19 | -15.94-10.61 | 0.077 (ST) |
| Waist circumference, cm | 516 | -2.03±8.00 | -2.00 | -43.00-45.00 | <0.001 (WSR) |
| Upper arm circumference, cm | 516 | -1.41±2.75 | -2.00 | -10.00-10.00 | <0.001 (WSR) |
| Triceps skinfold, cm | 516 | -0.39±0.63 | -0.35 | -3.00-1.20 | <0.001 (ST) |
| SBP, mmHg | 516 | -19.35±25.28 | -18 | -148.00-56.00 | <0.001 (ST) |
| DBP, mmHg | 516 | -13.35±25.29 | 15.72 | -80.00-30.00 | <0.001 (ST) |
| Heart rate, bpm | 493 | -10.30±12.90 | -10 | -104.00-22.00 | <0.001 (ST) |
| Daytime SBP, mmHg | 478 | -23.24±24.80 | -22.50 | -147.00-29.00 | <0.001 (ST) |
| Daytime DBP, mmHg | 478 | -21.45±17.98 | -22.00 | -86.00-24.00 | <0.001 (ST) |
| Night-time SBP, mmHg | 478 | -17.77±19.11 | -17.00 | -127.00-25.00 | <0.001 (ST) |
| Night-time DBP, mmHg | 478 | -19.07±15.89 | -18.00 | -81.00-14.00 | <0.001 (ST) |
BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; ST: sign test; WSR: Wilcoxon signed-rank test.
Table 2 shows changes in patients’ lifestyles. The reduction in alcohol consumption and the unexpected increase in regular exercise should be noted.
Changes in patients’ lifestyles between the initial and the final visits.
| n (%) | ||
|---|---|---|
| Initial | Final | |
| Smoking | ||
| Ex-smoker | 60 (11.6) | 90 (17.4) |
| Never-smoker | 389 (75.4) | 402 (77.9) |
| Current smoker | 67 (13.0) | 24 (4.7) |
| Alcohol consumption | ||
| Never | 57 (11.1) | 251 (48.7) |
| Occasional | 107 (20.8) | 161 (31.3) |
| Regular/moderate (one or two drinks/day) | 233 (45.2) | 68 (13.2) |
| Excessive (more than two drinks/day) | 80 (15.5) | 17 (3.3) |
| Alcoholic (more than three drinks daily) | 36 (7.0) | 8 (1.6) |
| Abstinent (no drinks for at least one year) | 2 (0.4) | 10 (1.9) |
| Exercise | ||
| Physically inactive | 371 (71.9) | 144 (28.0) |
| Physically active | 145 (28.1) | 371 (72.0) |
Table 3 reports the results of the biochemical assessments. Significant differences were found in almost every metabolic variable studied, including CRP, glycated hemoglobin, fructosamine, TC, HDL, LDL, very-low-density lipoprotein, triglycerides, fibrinogen, homocysteine, uric acid and microalbuminuria (p<0.001). In addition, fasting insulin and C-peptide values decreased between the first and the final observation, although without statistically significant differences. Lp(a) levels were also significantly reduced.
Changes in biochemical assessments between the initial and the final visits.
| n | Mean difference | Median difference | Range (min-max) | p (WSR) | |
|---|---|---|---|---|---|
| CRP, mg/dl | 508 | -0.53±1.02 | -0.10 | -7.8-7.9 | <0.001 |
| HbA1c, % | 484 | -0.96±1.54 | -0.40 | -7.6-3.5 | <0.001 |
| Fructosamine, mmol/l | 387 | -71.25±103.16 | -22.00 | -379-123 | <0.001 |
| Fasting insulin, μU/ml | 463 | -0.53±6.08 | 0.00 | -30-37.5 | 0.188 |
| C-peptide, ng/ml | 474 | -0.09±1.06 | 0.00 | -5.3-8.2 | 0.042 |
| TC, mg/dl | 516 | -103.68±63.02 | -101.00 | 308.00-88.00 | <0.001 |
| HDL, mg/dl | 516 | 19.35±12.19 | 19.00 | -74.00-60.00 | <0.001 |
| LDL, mg/dl | 505 | -75.60±40.80 | -77.00 | -224-59 | <0.001 |
| VLDL, mg/dl | 509 | -12.25±19.41 | -6.00 | -139-45 | <0.001 |
| Triglycerides, mg/dl | 508 | -69.77±114.52 | -42.50 | -968-144 | <0.001 |
| Fibrinogen, mg/dl | 477 | -64.43±73.99 | -59.00 | -412-292 | <0.001 |
| Homocysteine, mmol/l | 499 | -9.94±4.98 | -9.00 | -35-23 | <0.001 |
| Lp(a), mg/dl | 499 | -32.11±15.41 | -30.00 | -76-4.1 | <0.001 |
| ≤30 (normal) | 25 | ||||
| >30 (abnormal) | 484 | ||||
| Uric acid, mg/dl | 509 | -2.31±2.31 | -2.10 | -9.5-3.3 | <0.001 |
| 24-h microalbuminuria, μg/min | 489 | -29.87±50.94 | -8.00 | -283-80 | <0.001 |
CRP: C-reactive protein; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LP(a): lipoprotein(a); TC; total cholesterol; VLDL: very-low-density lipoprotein; WSR: Wilcoxon signed-rank test.
The cardiovascular exams performed included ECG, echocardiogram, IMT and ABPM.
Some changes were found in the ECG assessment. A normal exam was seen in 247 patients (47.2%) at the initial observation, compared to 281 patients (55.6%) at the final visit. Atrial fibrillation was present in four patients (0.8%) at the first consultation, and in 22 (4.4%) at the last. On echocardiographic study, alterations were seen in 267 patients (52.5%) at baseline, and in 273 patients (54.7%) at the end of the observation period. These included mild aortic valve abnormalities (mild regurgitation in two patients) and left atrial dilatation (four patients). Nevertheless, the overall improvement in these parameters should be noted. Concerning the 347 hypertensive patients (69.2%) who underwent ABPM, at the end 14 patients (2.9%) were found to have uncontrolled hypertension. The absence of acute episodes, such as MACE, during the study period should be noted.
Doppler ultrasound of the supra-aortic trunks showed an improvement in the number of patients with normal results from 82 patients (16.2%) to 100 (19.8%). IMT also improved, from an initial median of 2.90 mm to a final median of 1.40 mm (Wilcoxon signed-rank test, p<0.001). However, there was no reduction in the number of cases of vascular stenosis (McNemar test, p=0.500).
It should be noted that, of the 435 patients (85.4%) with hepatic steatosis, 208 (40.8%) presented hepatic steatosis with hepatomegaly (non-alcoholic fatty liver disease [NAFLD]).11 Nevertheless, liver function was only altered in a small number of patients (14.5%).
The kidney is one of the target organs of CVD. Our assessment of renal function showed that overall, 46.7% (241/516) of patients had abnormal creatinine clearance (mean 104.13±46.18 ml/min/m2, median 98.20; range 11.3-276.3), normal being defined as 70-135 ml/min/m2. Moreover, creatinine levels were abnormal in 31.8% (164/516) of patients, with mean levels of 87±0.34 g/dl (median 0.81; range 0.39-4.5), normal being defined as 0.66-1.25 g/dl. Mean creatinine level was 0.87±0.34 g/dl (median 0.81; range 0.39-4.5). Finally, 31% (160/516) of patients were found to have abnormal urea levels, normal being defined as 10-50 mg/dl. Mean urea level was 47.09±17.41 mg/dl (median 41.00 mg/dl; range 17.0-259.0).
Nosological groupsClinical diagnoses were classified into the following nosological groups: 390 patients with cardiovascular disease (75.6%), 50 with cerebrovascular disease (9.7%), 491 with metabolic diseases (95.2%) and 301 with behavioral diseases (58.3%).
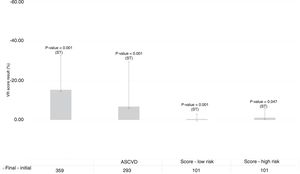
Stratification of cardiovascular riskInitial and final cardiovascular risk was stratified by estimating 10-year risk according to the parameters of the FRS, SCORE and ASCVD. Differences between cardiovascular risk scores calculated before and after treatment were as follows: FRS: -15.37 (min-max: -79.20-28.60), high-risk SCORE: -1.08 (min-max: -26.36-4.31), low-risk SCORE: -0.44 (min-max: -14.50-2.83) and ASCVD: -5.83 (min-max: -87.73-40.12). All improvements in cardiovascular risk scores between initial and final measures were statistically significant (p<0.05), regardless of the calculation method applied (Figure 1).
Pharmacological therapyThe pharmacological therapies administered to the patients are presented in Figure 2 and Table 4, divided into pharmacological groups. The following therapies were used most in this patient group: antidepressants (particularly selective serotonin reuptake inhibitors [SSRIs]), anxiolytics, lipid-lowering therapy including statins, and antiplatelets. Allopurinol, diuretics, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) were also commonly used. We also compared the efficacy of each of these therapies individually between patients who were receiving a particular treatment and those who were not, based on final Lp(a) levels. Results of the multiple regression analysis are shown in Supplementary Table 1. Briefly, statins, ACEIs, ARBs, oral antidiabetic drugs (OADs), antiplatelets, calcium channel blockers (CCBs) and allopurinol showed statistically significant differences on bivariate analysis (p<0.05) and were selected to enter the final model. This analysis excluded patients who died.
Associations between gender and pharmacological therapies and changes in Lp(a) levels between the initial and final measurement (excluding patients who died).
| Variables | n | Mean difference | Median difference | Range (min-max) | p |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 279 | -30.74±14.75 | -29.00 | -68.00-4.00 | 0.060 (MW) |
| Male | 202 | -33.50±16.17 | -32.00 | -71.00-4.10 | |
| Statins | |||||
| No | 38 | -18.33±12.70 | -18.00 | -60.00-4.10 | <0.001 (MW) |
| Yes | 443 | -33.06±15.07 | -31.00 | -71.00-4.00 | |
| ACEIs | |||||
| No | 222 | -27.89±14.87 | -25.00 | -68.00-4.10 | <0.001 (MW) |
| Yes | 259 | -35.33±15.05 | -34.00 | -71.00-0.00 | |
| ARBs | |||||
| No | 274 | -29.35±14.35 | -27.00 | -68.00-4.10 | <0.001 (MW) |
| Yes | 207 | -35.27±16.12 | -34.00 | -71.00-0.00 | |
| OADs | |||||
| No | 337 | -30.04±15.39 | -27.00 | -71.00-4.10 | <0.001 (MW) |
| Yes | 143 | -36.20±14.62 | -36.00 | -71.00--8.00 | |
| Antiplatelets | |||||
| No | 217 | -27.31± 14.84 | -24.40 | -70.00-4.10 | <0.001 (MW) |
| Yes | 263 | -35.73± 14.84 | -36.00 | -71.00-0.00 | |
| CCBs | |||||
| No | 290 | -29.00±14.74 | -27.00 | -70.00-4.10 | <0.001 (MW) |
| Yes | 191 | -36.29±15.39 | -35.00 | -71.00-0.00 | |
| Allopurinol | |||||
| No | 242 | -27.85±14.11 | -25.00 | -68.00-4.10 | <0.001 (MW) |
| Yes | 239 | -35.99±15.61 | -36.00 | -71.00-0.00 | |
| Antidepressants | |||||
| No | 102 | -30.23±14.59 | -27.00 | -71.00-4.10 | 0.303 (MW) |
| Yes | 379 | -32.34±15.60 | -30.00 | -71.00-4.00 | |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CCBs: calcium channel blockers; MW: non-parametric Mann-Whitney test; OAD: oral antidiabetic drugs; SD: standard deviation.
Overall, the use of statins, ACEIs, ARBs, OADs, antiplatelets, CCBs and allopurinol was associated with greater reductions in Lp(a) values during the follow-up period. The final optimized regression model showed that the regression coefficient for reduction in Lp(a) levels in patients under statins between the initial and the final measurement was more than 10.635, compared to those not under statins, after adjustment for antiplatelets, allopurinol and antidepressants (final model variables). Furthermore, the regression coefficient for reduction in Lp(a) levels in patients under antiplatelets was more than 4.786, compared with those not under antiplatelets, after adjustment for statins, allopurinol and antidepressants. The regression coefficient for reduction in Lp(a) levels in patients under allopurinol was more than 5.376, in comparison with those who were not under allopurinol, after adjustment for statins, antiplatelets and antidepressants. Finally, the regression coefficient for reduction in Lp(a) levels in patients under antidepressants was more than 4.367 compared with those who were not under antidepressants, after adjustment for statins, antiplatelets and allopurinol.
DiscussionIn this retrospective observational study, we appraised the effects of using combined drug therapies on changes in Lp(a) levels in patients with high cardiovascular risk, without MACE. Overall, our results indicate significant clinical improvements between the initial and final measurements, especially regarding blood pressure and metabolic risk factors.
Neither social nor demographic factors appeared to pose difficulties in following the proposed treatments and guidance for improving lifestyles. The improvements in blood pressure control and significant reductions in waist circumference should be noted, as these are important cardiovascular risk factors influencing the atherosclerotic process.
This study demonstrated a significant correlation between Lp(a) levels and cardiovascular risk scores in patients with high cardiovascular risk but without MACE. In previous work, we found that increased Lp(a) levels in these individuals were strongly associated with cardiovascular risk factors such as IMT, LDL-C and homocysteine, as well as with NAFLD.12 In addition, for the same population, a positive and significant correlation was found between Lp(a) and the risk scores used for CVD stratification (p<0.001).12 These data suggest that guidelines for assessing the severity of the atherosclerotic process should be reviewed.13
Until recently, Lp(a) has been a recognized but under-appreciated cardiovascular risk factor, largely because a therapeutic approach has yet to be established.14,15 As specific treatments are lacking, Lp(a) warrants further investigation.1 Early primary prevention is recommended, with the introduction of available therapies in clinically stable patients, regardless of vascular age risk (personal, family and lifestyle) and metabolic alterations promoting the atherosclerosis process. However, currently used cardiovascular risk scores do not fit this interventional approach, but rather delay the administration of combined drug treatments. Patients’ commitment to implementing changes, including adopting healthier lifestyles,16–18 such as regular exercise, is essential to the success of the treatment.8,19
Lp(a) acts as a marker of severity and progression of CVD in patients at particular risk for poor outcomes.20 In our study, although there was a significant decrease in IMT, cases of carotid stenosis persisted after the treatment period.20 Therefore, although this was not evident in previous studies, lipid-lowering therapy, especially statins combined with other treatments, appears to be involved in Lp(a) reduction. Similar results were obtained with ACEIs, diuretics, ARBs, CCBs and antiplatelets. The effects of allopurinol, OADs and proton pump inhibitors in combined drug therapy, should also be highlighted. On the other hand, beta-blockers and insulin had less effect.
These results confirm that the role of Lp(a) as a biomarker of cardiovascular risk should be analyzed along with that of patients’ lifestyles and biochemical parameters. Lp(a) may thus be a crucial indicator for comprehensive early multidisciplinary treatment, directed at all cardiovascular risk factors and associated comorbidities.
A large proportion of patients had moderate renal failure, as expected since the kidney is a target organ for atherosclerotic disease, and also due to cellular senescence. This finding indicates that appropriate adjustments to therapy may be required. We also observed a high number of patients with hepatic steatosis, which indicates that this condition should be considered in the presence of NAFLD and, as such, may represent a way to contextualize the evolution and severity of atherosclerotic disease.11,21
In view of the clinically relevant and statistically significant reduction of Lp(a) seen in our study,22 further research is required to establish a consistent therapeutic strategy to achieve this end,23 as well as to improve upon the subjective calculation of cardiovascular risk with currently used algorithms. Nonetheless, we can state that first-line therapies should include statins,24,25 allopurinol, antiplatelets and antidepressant drugs. In addition, the atherogenicity of Lp(a) may be modified through substantial reductions in LDL levels.
Although clinically relevant, our results should be interpreted in the light of the inherent methodological limitations of the study. As it is based on data from a single center, the results cannot be extrapolated to the Portuguese population in general. Due to its retrospective nature, the study could have been affected by information bias from data gathered from patients’ charts, including changes in therapeutic guidelines over the long follow-up period (median 11.0 years). Furthermore, it was not possible to establish temporal relationships between risk factors and CVD that demonstrate causation.
ConclusionLp(a) is a key indicator of global cardiovascular risk and should be considered a therapeutic target. According to the results obtained for our patients, it may be important to initiate primary prevention, including combined drug therapies, while addressing all cardiovascular risk factors, to delay the atherosclerotic process. First-line therapy should include statins, antiplatelets, allopurinol and antidepressants, particularly SSRIs. Among other pharmacological therapies, CCBs, ACEIs, OADs and ARBs should also be considered, as well as lifestyle modifications.
Conflicts of interestThe author has no conflicts of interest to declare.









 FRS: Framingham risk score;
FRS: Framingham risk score;  ACEI: angiotensin-converting enzyme inhibitor;
ACEI: angiotensin-converting enzyme inhibitor; 


