We sought to assess the prognostic impact of left atrial (LA) size on long-term outcomes of ST-segment elevation myocardial infarction (STEMI).
MethodsWe studied 200 consecutive patients admitted to a single center between January 2010 and December 2014 with non-fatal STEMI treated with primary percutaneous coronary intervention (pPCI) who underwent a comprehensive echocardiographic examination at discharge. LA volume was estimated by the area-length method. The left atrium was classified as normal, mildly, moderately or severely enlarged by LA volume index (LAVI). The endpoints were defined as all-cause mortality, a cardiac composite endpoint (all-cause mortality, reinfarction, unplanned revascularization and hospitalization for heart failure) and a cardiovascular composite endpoint (cardiac endpoint plus atrial fibrillation and ischemic stroke) during follow-up.
ResultsIn this STEMI population, 58% had normal LA size, 22.5% had mild LA enlargement, 10% had moderate LA enlargement and 9.5% had severe LA enlargement. During a median follow-up of 28 (IQR 21-38) months, 14 (7.0%) patients died, 53 (26.5%) had the cardiac and 58 (29%) the cardiovascular composite endpoints. There was a stepwise increase in the incidence of all-cause mortality (p=0.020) and both cardiac (p<0.001) and cardiovascular (p<0.001) endpoints with each increment of LAVI class. In multivariate analysis, severe LA enlargement by LAVI was an independent predictor of all-cause mortality (HR: 11.153; 95% CI: 1.924-64.642, p=0.007) and the cardiac (HR: 4.351; 95% CI: 1.919-9.862, p<0.001) and cardiovascular (HR: 4.351; 95% CI: 1.919-9.862, p<0.001) endpoints during follow-up.
ConclusionsThis contemporary study confirms the prognostic effect of LA size at discharge, applying the most recent reference values in STEMI patients treated with pPCI.
Este estudo procurou avaliar o impacto prognóstico da dimensão da aurícula esquerda (AE) no enfarte agudo do miocárdio com supradesnivelamento do segmento ST (EAMCSST).
MétodosForam estudados 200 doentes consecutivos, admitidos num único centro por EAMCSST não fatal, submetidos a intervenção coronária percutânea primária (ICPp) entre janeiro de 2010 e dezembro de 2014, que realizaram ecocardiograma à alta. O volume da AE foi calculado pelo método area-length. A AE foi classificada como normal ou ligeira, moderada ou severamente dilatada pelo volume indexado. Os endpoints primários foram a mortalidade por todas as causas, um endpoint composto cardíaco (morte, re-enfarte, revascularização não planeada e admissão por insuficiência cardíaca) e outro cardiovascular (endpoint cardíaco, fibrilhação auricular e acidente vascular cerebral isquémico) no follow-up.
ResultadosEm 58% dos doentes a AE tinha dimensões normais e 22,5% apresentavam dilatação ligeira, 10% dilatação moderada e 9,5% dilatação severa. Durante um follow-up mediano de 28(IIQ 21-38) meses, 14 (4%) doentes morreram, 53(26,5%) tiveram o endpoint composto cardíaco e 58 (29%) o endpoint composto cardiovascular. A incidência de morte (p=0,020) e dos endpoints compostos cardíaco (p<0,001) e cardiovascular (p<0,001) no follow-up foi superior nos maiores graus de dilatação da AE. Na análise multivariada, a dilatação severa da AE foi preditora independente de morte (HR: 11,153; 95% CI: 1,924-64,642, p=0,007) e dos endpoints compostos cardíaco (HR: 4,351; 95% CI: 1,919-9,862, p<0,001) e cardiovascular (HR: 4,351; 95% CI: 1,919-9,862, p<0,001).
ConclusõesEste estudo confirma a importância prognóstica do tamanho da AE na alta utilizando os valores de referência mais recentes nos EAMCSST submetidos a ICPp.
The left atrium modulates left ventricular (LV) filling and cardiovascular performance, acting as a reservoir, a conduit and a contractile pump during the cardiac cycle. Left atrial (LA) size is a marker of LV filling pressure and reflects the severity and chronicity of diastolic dysfunction in those without atrial fibrillation (AF) and significant valvular disease.1,2 Unlike other Doppler variables of LV diastolic function affected by acute hemodynamic changes, it is a stable parameter that combines the effects of chronic cardiovascular conditions and acute disease.1
Previous studies have demonstrated that LA size as determined by echocardiography is a strong predictor of AF, heart failure (HF), stroke and death in the general population and in patients with cardiovascular disease.2-17 In acute coronary syndrome (ACS), it has been shown that LA enlargement on admission is associated with HF, cardiac mortality and all-cause mortality during follow-up, providing additional prognostic information to other clinical and echocardiographic data.18–24
LA volume index (LAVI) is the recommended parameter for echocardiographic assessment of LA size.25 However, more easily performed measurements, such as anteroposterior (AP) diameter and area by planimetry in 4-chamber apical view, are still widely used in clinical practice.
We sought to assess the prognostic impact of LA enlargement by LAVI on long-term outcomes of ST-segment elevation myocardial infarction (STEMI).
MethodsStudy populationWe studied 299 consecutive patients admitted to a single center between January 2010 and December 2014 with STEMI treated with primary percutaneous coronary intervention (pPCI). ST-segment elevation was defined according to current guidelines.26 Patients who died during hospitalization (n=32), those who did not undergo a comprehensive echocardiographic study (n=17), and those without follow-up (n=14) were excluded. Patients with AF (n=30), mitral stenosis (n=1), prosthetic mitral valves (n=0), severe valvular heart disease (n=1) and heavy mitral calcification (n=4) were also excluded. Thus, we studied 200 patients with non-fatal STEMI treated with pPCI.
Echocardiographic parametersEchocardiography was performed a median of five (interquartile range [IQR] 4-6) days after admission. Studies were performed by experienced sonographers and reviewed by staff cardiologists. The scanners used were a Philips iE33 and Philips CX50 with an S5-1 probe and a GE Vivid S6 with a 3Sc-RS probe.
LA volume was estimated by the area-length method. LA area and length were measured at end-systole in apical 4- and 2-chamber views, excluding the atrial appendage and pulmonary veins. LA volume was calculated using the formula (A1×A2/L)×8/3π, where A1 and A2 are the LA areas from 4- and 2-chamber views, respectively, and L is the shorter of the two long-axis lengths. LA volume was indexed to body surface area to produce LAVI. In accordance with current guidelines,25 normal LA size was defined as LAVI ≤34 ml/m2, mild dilatation as 35-41 ml/m2, moderate dilatation as 42-48 ml/m2 and severe dilatation as >48 ml/m2.
Interventricular septal and posterior wall thickness and end-systolic and end-diastolic LV diameters were measured in parasternal long-axis view. LV mass was computed by the cube formula. LV end-diastolic diameter and mass were indexed to body surface area.25 Mitral regurgitation was identified by color flow imaging. Mild regurgitation was graded with color flow imaging, and more than mild mitral regurgitation was also categorized by the vena contracta or proximal isovelocity surface area (PISA) methods. Mitral inflow was assessed in apical 4-chamber view, using a pulsed-wave Doppler beam aligned parallel to the flow direction and with the sample volume at the leaflet tips. From the mitral inflow profile, peak E- and A-wave velocities and E-wave deceleration time were measured and the E/A ratio was calculated. Early mitral valve annulus diastolic (E’) and systolic (S’) velocities were measured by Doppler tissue imaging and the E/E’ ratio was computed, except in those with heavy mitral calcification. LV diastolic function was classified as normal, grade I, II or III by combining the E/A ratio, E/E’ ratio and LAVI. LV ejection fraction (LVEF) was calculated from apical 4- and 2-chamber views by the biplane Simpson method. Tricuspid annular plane systolic excursion was measured with the M-mode cursor aligned along the direction of the lateral tricuspid annulus in apical 4-chamber view.
Other clinical variablesData on demographics, previous medical history, clinical presentation, coronary artery disease (CAD) extent, culprit artery, laboratory findings and in-hospital procedures were also collected from medical charts.
Study endpointsFollow-up was performed by reviewing hospital medical charts. Patients were followed for a median of 24 (IQR 13-34) months. The endpoints were defined as all-cause mortality, a cardiac composite endpoint of all-cause mortality, reinfarction, unplanned revascularization and hospitalization for HF, and (as LA size is an independent predictor of AF and ischemic stroke in the general population) a cardiovascular composite endpoint of all-cause mortality, reinfarction, unplanned revascularization, hospitalization for HF, AF and ischemic stroke.
Statistical analysisBaseline and presentation characteristics, CAD extent, echocardiographic parameters, cardiac biomarkers, incidence of long-term ACS complications and of the endpoints were compared between patients with normal LA size and mild, moderate and severe LA enlargement by LAVI. Continuous variables with normal distribution were expressed as mean (standard deviation) or as median (IQR) if they did not have a normal distribution. Categorical variables were presented as frequencies and proportions. Comparison between continuous variables was computed with analysis of variance or with the independent-samples Kruskal-Wallis test if they did not have a normal distribution. The chi-square test was used to compare categorical variables.
The log-rank test was used to compare all-cause mortality and cardiovascular event rates between degrees of LAVI enlargement. A sensitivity analysis was performed to assess the robustness of the results by comparing all-cause mortality and the two composite endpoint rates between moderate/severe LA enlargement (joined in one group) and normal/mild enlargement (joined in another group) using the log-rank test. Multivariate Cox regression analysis was used to assess the independent association between LA size and all-cause mortality and the two composite endpoints. Variables associated with the endpoints in univariate analysis were included in the models.
Two-tailed tests were used to calculate p-values. A p-value of <0.05 was considered significant. All statistics were performed using IBM SPSS software, version 20.0.0.
ResultsIn this STEMI population, 57.4% had normal LA size, 23.5% had mild LA enlargement, 9.8% had moderate enlargement and 9.3% had severe enlargement. The baseline and in-hospital characteristics and echocardiographic parameters according to degree of LA enlargement are shown in Table 1. Baseline characteristics were similar between groups, except for the higher prevalence of previous coronary artery bypass grafting surgery in patients with greater LA enlargement. Angiography at pPCI showed similar CAD severity between groups. Patients with greater LA enlargement had higher LV end-diastolic diameter index, mass index and E/E’ ratio. Worse LV diastolic dysfunction was associated with greater LA enlargement. At discharge, therapy with aspirin, P2Y12 inhibitors, statins, beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers was similar between groups (Supplementary Table 1).
Baseline, in-hospital and echocardiographic characteristics and discharge medical therapy according to left atrial enlargement by volume index.
| Normal LA size | Mild enlargement | Moderate enlargement | Severe enlargement | p | |
|---|---|---|---|---|---|
| n=116 | n=45 | n=20 | n=19 | ||
| Baseline characteristics | |||||
| Age, years | 62±13 | 64±14 | 64±11 | 68±11 | 0.274 |
| Male gender | 70.7% | 68.8% | 85.0% | 73.7% | 0.606 |
| BMI, kg/m2 | 27.0±4.1 | 27.0±4.1 | 25.6±3.0 | 28.1±8.5 | 0.412 |
| Hypertension | 58.6% | 55.6% | 70.0% | 68.4% | 0.631 |
| Diabetes | 28.4% | 20.0% | 25.0% | 31.6% | 0.689 |
| Dyslipidemia | 56.9% | 41.7% | 50.0% | 57.9% | 0.525 |
| Current smoker | 28.4% | 33.3% | 50.0% | 21.1% | 0.211 |
| Previous PCI | 3.4% | 13.3% | 10.0% | 0.0% | 0.064 |
| Previous CABG | 0.0% | 0.0% | 5.0% | 5.3% | 0.037 |
| In-hospital characteristics | |||||
| Symptoms-to-PCI time, hours (mean ± SD) | 6.0 (4.0-9.0) | 6.0 (4.0-10.0) | 6.5 (4.0-10.0) | 6.0 (5.0-12.0) | 0.812a |
| Culprit vessel | |||||
| Left main | 0.9% | 0.0% | 0.0% | 0.0% | 0.214 |
| LAD | 50.9% | 42.2% | 65.0% | 36.8% | |
| Circumflex | 10.3% | 17.8% | 0.0% | 5.3% | |
| Right coronary | 37.9% | 40.0% | 35.0% | 52.6% | |
| Bypass graft | 0.0% | 0.0% | 0.0% | 5.3% | |
| Multivessel disease | 68.1% | 80.0% | 75.0% | 78.9% | 0.444 |
| Killip class >II | 11.3% | 4.4% | 15.0% | 15.8% | 0.328 |
| Biomarkers | |||||
| Peak CK, IU/l | 1693 (769-3118) | 1937 (782-4117) | 2603 (873-5109) | 3069 (981-4135) | 0.259 |
| Peak NT-proBNP, pg/ml | 1335 (584-3546) | 1038 (344-3116) | 1980 (281-4920) | 2080 (1031-5243) | 0.587a |
| Echocardiographic characteristics | |||||
| LVEDD index, mm | 28.0±4.1 | 28.4±5.1 | 30.3±2.8 | 28.5±4.3 | 0.015 |
| LVMI, g/m2 | 89.5±16.7 | 98.6±22.6 | 98.2±11.6 | 95.4±22.7 | 0.019 |
| LVEF, % | 48.8±8.9 | 47.0±9.0 | 44.4±10.3 | 44.5±11.6 | 0.089 |
| Mitral S’ wave, cm/s | 6.3±1.1 | 6.1±1.3 | 5.6±1.2 | 5.7±1.3 | 0.077 |
| Mitral E wave, cm/s | 63.4±19.8 | 67.4±23.4 | 77.4±18.7 | 75.9±26.3 | 0.012 |
| Mitral A wave, cm/s | 75.1±18.8 | 74.5±21.6 | 59.8±21.8 | 60.3±25.2 | 0.002 |
| Mitral E/A | 0.9±0.4 | 1.0±0.6 | 1.5±0.8 | 1.3±0.6 | <0.001 |
| Mitral deceleration time, ms | 201.8±52.4 | 199.2±56.8 | 177.4±46.1 | 212.9±68.9 | 0.230 |
| Mean mitral E/E’ | 11.4±4.4 | 13.0±5.7 | 15.7±8.8 | 13.9±5.3 | 0.015 |
| LV diastolic function | |||||
| Normal | 57.84 | 4.5% | 0.0% | 0.0% | <0.001 |
| Grade I dysfunction | 27.8% | 29.5% | 0.0% | 0.0% | |
| Grade II dysfunction | 13.9% | 61.4% | 85.0% | 75.0% | |
| Grade III dysfunction | 0.9% | 4.5% | 15.0% | 25.0% | |
| Mitral regurgitation | |||||
| No regurgitation | 66.7% | 54.2% | 20.0% | 15.8% | <0.001 |
| Mild | 26.5% | 37.5% | 65.0% | 73.7% | |
| Moderate | 6.8% | 8.3% | 15.0% | 10.5% | |
| Tricuspid S’ wave, cm/s | 11.6±2.0 | 12.5±2.1 | 11.6±2.6 | 11.3±3.0 | 0.170 |
BMI: body mass index; CABG: coronary artery bypass grafting; LA: left atrial; LAD: left anterior descending; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; PCI: percutaneous coronary intervention; SD: standard deviation.
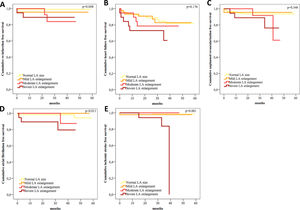
During follow-up, 14 (7%) patients died, 53 (26.5%) had the cardiac composite endpoint of all-cause mortality, reinfarction, unplanned revascularization and hospitalization for HF, and 58 (29.0%) had the cardiovascular composite endpoint of all-cause mortality, reinfarction, unplanned revascularization, hospitalization for HF, AF and ischemic stroke. Table 2 presents the incidence of cardiovascular events during follow-up and Supplementary Figure 1 shows Kaplan-Meier curves for cardiovascular events during follow-up. Patients with greater LA enlargement had higher incidence of reinfarction, AF and ischemic stroke during follow-up. The proportion of patients with unplanned revascularization or HF hospitalization during follow-up was not significantly different between groups, but there was a trend for a higher proportion of unplanned revascularization and hospitalization for HF during follow-up in patients with higher LAVI. Figure 1 shows the Kaplan-Meier survival curves for all-cause mortality and composite endpoints during follow-up according to LA size. There was a stepwise increase in the incidence of all-cause mortality and the two composite endpoints with each increase in LAVI class. Kaplan-Meier curves also showed a significant difference in the incidence of all-cause mortality (p=0.003) and both cardiac and cardiovascular composite endpoints (p<0.001 for both) when moderate-to-severe LA enlargement was compared to normal size or mild enlargement (Supplementary Figure 2). In multivariate analysis, moderate and severe LAVI enlargement and age >75 years were independent predictors of all-cause mortality during follow-up (Table 3). Severe LA enlargement, age >75 years, LV hypertrophy, moderate-to-severe LV systolic dysfunction and moderate mitral regurgitation were independent predictors of the cardiac and cardiovascular composite endpoints during follow-up (Tables 4 and 5). Severe LA enlargement remained an independent predictor of the two composite endpoints after adjustment for mean E/E’ >14.
Cardiovascular events and all-cause mortality during follow-up according to left atrial enlargement by volume index.
| Total | Normal LA size | Mild LA enlargement | Moderate LA enlargement | Severe LA enlargement | p | |
|---|---|---|---|---|---|---|
| n=200 | n=116 | n=45 | n=20 | n=19 | ||
| Reinfarction | 7 | 0.9% | 4.4% | 10% | 10.5% | 0.018 |
| Unplanned revascularization | 14 | 6.0% | 4.4% | 10.0% | 15.8% | 0.280 |
| Hospitalization for HF | 31 | 12.9% | 13.3% | 20.0% | 31.6% | 0.197 |
| Atrial fibrillation | 7 | 2.6% | 0.0% | 5.0% | 15.8% | 0.021 |
| Ischemic stroke | 5 | 0.9% | 2.2% | 0.0% | 15.8% | 0.016 |
| All-cause mortality | 14 | 3.4% | 6.7% | 15.0% | 21.2% | 0.014 |
| Cardiac composite endpoint | 53 | 19.8% | 22.2% | 45.0% | 57.9% | 0.001 |
| Cardiovascular composite endpoint | 58 | 22.4% | 22.2% | 50.0% | 63.2% | <0.001 |
Cardiac composite endpoint: all-cause mortality, reinfarction, unplanned revascularization and hospitalization for heart failure; Cardiovascular composite endpoint: all-cause mortality, reinfarction, unplanned revascularization, hospitalization for heart failure, atrial fibrillation and ischemic stroke; HF: heart failure; LA: left atrial.
Multivariate Cox regression analysis for all-cause mortality during follow-up.
| HR | 95% CI | P | |
|---|---|---|---|
| Age | |||
| 66-75 yearsa | 1.320 | 0.203-8.569 | 0.771 |
| >75 yearsa | 10.390 | 2.556-42.240 | 0.001 |
| LA enlargement | |||
| Mildb | 1.294 | 0.212-7.883 | 0.780 |
| Moderateb | 7.397 | 1.192-45.897 | 0.032 |
| Severeb | 11.153 | 1.924-64.642 | 0.007 |
| LV hypertrophy | 1.371 | 0.390-4.648 | 0.623 |
| Moderate-to-severe LV systolic dysfunction | 1.927 | 0.558-6.657 | 0.300 |
| Mitral regurgitationc | |||
| Mildd | 0.409 | 0.089-1.870 | 0.249 |
| Moderated | 1.088 | 0.189-6.276 | 0.925 |
CI: confidence interval; HR: hazard ratio; LA: left atrial; LV: left ventricular.
Multivariate Cox regression analysis for the cardiac composite endpoint of all-cause mortality, reinfarction, unplanned revascularization and hospitalization for heart failure during follow-up.
| Model A | Model Ba | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| 66-75 yearsb | 0.821 | 0.405-1.663 | 0.584 | 0.493 | 0.196-1.237 | 0.132 |
| >75 yearsb | 1.910 | 0.963-3.788 | 0.064 | 1.255 | 0.465-3.389 | 0.564 |
| LA enlargement | ||||||
| Mildc | 0.733 | 0.323-1.663 | 0.458 | 0.692 | 0.252-1.904 | 0.476 |
| Moderatec | 1.904 | 0.825-4.391 | 0.131 | 1.614 | 0.625-4.168 | 0.322 |
| Severec | 4.351 | 1.919-9.862 | <0.001 | 4.534 | 1.604-12.812 | 0.004 |
| LV hypertrophy | 2.279 | 1.269-4.093 | 0.006 | 2.018 | 0.966-4.213 | 0.062 |
| Moderate-to-severe LV systolic dysfunction | 3.080 | 1.689-5.615 | <0.001 | 2.793 | 1.349-5.784 | 0.006 |
| Mitral regurgitationd | ||||||
| Milde | 0.863 | 0.424-1.758 | 0.685 | 1.001 | 0.399-2.512 | 0.999 |
| Moderatee | 2.992 | 1.290-6.938 | 0.011 | 6.744 | 2.186-20.812 | 0.001 |
| Mean E/E’ >14 | 1.679 | 0.802-3.518 | 0.168 | |||
CI: confidence interval; HR: hazard ratio; LA: left atrial; LV: left ventricular.
Multivariate Cox regression analysis for the cardiovascular composite endpoint of all-cause mortality, reinfarction, unplanned revascularization, hospitalization for heart failure, atrial fibrillation and ischemic stroke during follow-up.
| Model A | Model Ba | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| 66-75 yearsb | 0.969 | 0.500-1.877 | 0.969 | 0.683 | 0.285-1.634 | 0.391 |
| >75 yearsb | 1.923 | 0.977-3.786 | 0.058 | 1.350 | 0.510-3.511 | 0.538 |
| LA enlargement | ||||||
| Mildc | 0.707 | 0.320-1.561 | 0.390 | 0.633 | 0.239-1.672 | 0.356 |
| Moderatec | 1.806 | 0.809-4.034 | 0.149 | 1.382 | 0.561-3.406 | 0.482 |
| Severec | 4.426 | 1.980-9.892 | <0.001 | 4.085 | 1.548-10.783 | 0.004 |
| LV hypertrophy | 1.905 | 1.095-3.313 | 0.022 | 1.641 | 0.832-3.236 | 0.153 |
| Moderate-to-severe LV systolic dysfunction | 2.847 | 1.620-5.006 | <0.001 | 2.534 | 1.288-4.983 | 0.007 |
| Mitral regurgitationd | ||||||
| Milde | 0.741 | 0.371-1.482 | 0.397 | 0.882 | 0.371-2.096 | 0.776 |
| Moderatee | 2.372 | 1.057-5.324 | 0.036 | 4.813 | 1.677-13.811 | 0.003 |
| Mean E/E’ >14 | 1.361 | 0.669-2.766 | 0.395 | |||
CI: confidence interval; HR: hazard ratio; LA: left atrial; LV: left ventricular.
The present study demonstrates that severe LA enlargement by LAVI measured by the area-length method was an independent predictor of all-cause mortality and the cardiac and cardiovascular composite endpoints during follow-up. Also, greater LA enlargement was associated with worse outcomes during follow-up.
Previous studies have demonstrated the prognostic significance of LA size in the general population and in patients with cardiomyopathy, CAD and valvular heart disease. In the general population, LA size measured by AP diameter, area or volume index is an independent predictor of first congestive HF episode, myocardial infarction (MI), revascularization, AF, stroke and all-cause mortality.2–6,9,10,12,27,28 In those with HF and depressed LVEF, LA size is associated with cardiovascular hospitalization and death independently of age, New York Heart Association functional class, LVEF and diastolic function.7,8,11,13,14 LA enlargement has also been associated with poor outcomes in patients with CAD.16,17 In the setting of ACS, LAVI computed by the area-length method or the biplane method of disks is an independent predictor of death, reinfarction, unplanned revascularization, HF and AF during follow-up.18–24 This contemporary study extends the previous conclusions of the prognostic implication of LA enlargement to STEMI patients, using the most recent reference values of two-dimensional (2D) LAVI to categorize dilatation. The increased risk associated with LA size is shown to be continuous, and greater LA enlargement is associated with worse outcome.
After MI, complex LV structural and functional modifications cause LV diastolic dysfunction, increased LV end-diastolic pressures and LA remodeling.22,29,30 The rate of LA remodeling appears to be more significant during the early phase as a subacute maladaptive reaction to an acute event.30 In the present study, the echocardiographic examination was performed at discharge, thus reflecting initial LV and LA remodeling. It has been shown that LA enlargement at discharge is a more accurate prognostic marker than at admission and that greater increases in LA size during hospitalization are associated with worse outcomes.21
There are numerous methods for assessing LA size. LA AP diameter and area have been widely used in clinical practice and research. However, LA geometry and remodeling are complex and a single-dimensional view may not accurately represent actual LA size.25 The recommended method to assess LA size is therefore by calculating biplane LAVI.25 A previous study demonstrated that LAVI is a more robust cardiovascular risk marker than AP diameter or area in the general population with sinus rhythm.3 The present study shows that biplane LAVI is a significant predictor of cardiovascular events in STEMI patients without AF or significant mitral stenosis. It has been shown that three-dimensional (3D) LAVI is more accurate and provides better prognostic information than 2D volumes.11,31–35 However, 3D echocardiography is not widely available and depends on image quality and patient cooperation, and there are no established reference values.25
This study also shows that patients with more severe LA enlargement also have a greater degree of LV diastolic dysfunction and higher E/E’ ratio, a marker of elevated LV filling pressures. It has been demonstrated that diastolic dysfunction is a predictor of long-term prognosis in the setting of ACS. Previous studies showed that a restrictive LV filling pattern and E/E’ ≥15 were independently associated with death and HF.36–39 However, in this study only moderate and severe LA enlargement and age >75 years were predictors of all-cause mortality and only severe LA enlargement, moderate-to-severe LV systolic dysfunction, LV hypertrophy and moderate mitral regurgitation were independent predictors of both composite endpoints. Severe LA enlargement remained an independent predictor of the two composite endpoints after adjustment for mean E/E’ >14. LA size reflects the chronicity of exposure to elevated LV filling pressures and provides prognostic information beyond degree of diastolic function, which is determined from multiple load-dependent parameters.1 This may be particularly relevant in the context of an acute event like STEMI treated with pPCI, in which multiple hemodynamic changes occur.
The study has several limitations. Use of the area-length technique to estimate LAVI assumes an ellipsoidal LA shape, which may result in inaccurate volume estimation.40 However, there is a large body of evidence on this method as a prognostic marker. Also, LA shape and structure are complex, and so 3D echocardiography may be a more accurate method of assessing volume. Furthermore, in this study, we only assessed the prognostic significance of maximum LA volume. LA function can provide new insights into the importance of LA performance and more recent studies suggest that these parameters provide additional prognostic information.41–43 Finally, the sample size was relatively small and the number of events during follow-up was low, although the inclusion of endpoints composed of multiple variables, with a higher event rate, improved the statistical power of this study and avoided type II error. A larger population and longer follow-up period could have enabled more definite conclusions.
ConclusionsThis contemporary study confirms the prognostic effect of LA enlargement at discharge, applying the most recent reference values in patients with STEMI treated with pPCI. LA size was associated with LV dimension, hypertrophy, diastolic function and filling pressures in STEMI patients, reflecting LV remodeling. These data suggest that LAVI should be routinely assessed after STEMI, as it provides additional information to other prognostic markers, such as LVEF. This simple, easy and quickly performed measurement can be used as a tool for risk stratification and as a guide for increased surveillance after STEMI.
Conflicts of interestThe authors have no conflicts of interest to declare.