Left atrial (LA) size and function are associated with outcome after myocardial infarction (MI). In this study we aimed to assess the impact of LA function as a predictor of exercise capacity through speckle tracking echocardiography.
MethodsA total of 94 patients (mean age 54.8±11.0 years; 82% male) were enrolled one month after MI. Echocardiography was used to assess LA volumes and various indices of LA conduit, contraction and reservoir function. LA deformation was assessed by two-dimensional speckle tracking to calculate strain and strain rate at different phases of the cardiac cycle. Exercise capacity was assessed by oxygen uptake (VO2) on cardiopulmonary exercise testing.
ResultsIncreased LA volumes, especially LA volume before atrial contraction, were correlated with reduced peak VO2 and reduced VO2 at anaerobic threshold. Decreased peak VO2 was associated with reduced LA conduit function (ρ=0.24; p=0.02), but not with LA booster function (ρ=-0.07; p=0.53). Lower peak atrial longitudinal strain was associated with worse exercise capacity (ρ=0.24; p=0.02).
ConclusionsAfter MI, increased LA volumes were markers of decreased functional capacity that was associated with decreased LA conduit function, but not with LA contractile function. In these patients, LA longitudinal strain analysis may be useful to predict reduced exercise capacity.
As dimensões e a função da aurícula esquerda (AE) são determinantes independentes de prognóstico após enfarte do miocárdio (EAM). Neste estudo pretendemos avaliar o impacto da função da AE, avaliada por speckle tracking, enquanto preditor da capacidade funcional.
MétodosForam incluídos 94 doentes consecutivos (idade média: 54,8±11,0; 82% sexo masculino) um mês após o EAM. Os volumes da AE e os índices de função de condução, contração e reservatório da AE foram avaliados por ecocardiografia. O strain da AE foi avaliado por speckle tracking 2D ao longo das várias fases do ciclo cardíaco. A capacidade funcional foi avaliada por prova de esforço cardiopulmonar com avaliação do consumo de O2 (VO2).
ResultadosOs volumes da AE, especialmente o volume antes da contração auricular, correlacionaram-se com menor VO2 pico e menor VO2 no limiar anaeróbio. A diminuição do VO2 pico associou-se a menor função de condução (ρ=0.24; p=0.02), mas não com a função de contração da AE (ρ=-0.07; p=0,53). A capacidade funcional correlacionou-se com o strain longitudinal global da AE (ρ=0,24; p=0.02).
ConclusõesApós EAM, os volumes da AE são preditores de diminuição da capacidade funcional. Esta associou-se principalmente à diminuição da função de condução da AE, mas não com a função de contração. Além disso, a análise do strain longitudinal global da AE pode ser útil enquanto preditor da capacidade de exercício nestes doentes.
Reduced exercise capacity is frequent after myocardial infarction (MI) and is an important predictor of cardiovascular outcomes and decreased quality of life.1 However, the cardiac and non-cardiac mechanisms that underlie poor functional capacity after MI are not fully understood.
Left atrial (LA) size and function are important prognostic markers in several cardiovascular diseases, particularly after MI.2,3 The left atrium plays an important role in the regulation of global cardiac function, acting as a blood reservoir during systole, as a conduit during early diastole, and as an active pump during late diastole.4 However, beyond their prognostic value, few data are available regarding the impact of LA volume and function on exercise capacity. Assessment of strain and strain rate by two-dimensional speckle tracking analysis is a relatively new echocardiographic tool that tracks the pattern of myocardial speckles, frame by frame, in standard B-mode images. Several studies have shown good feasibility and reproducibility for assessment of the different phases of LA function by speckle tracking.5–8
Exercise capacity can be objectively assessed by cardiopulmonary exercise testing (CPET), in which oxygen uptake (VO2), carbon dioxide production, and ventilatory response are measured simultaneously. CPET provides the most accurate, reliable and reproducible measurements of exercise tolerance.1
The aim of this study was to assess the role of LA volumes and different indices of LA conduit, contraction and reservoir function, derived from both standard volumetric and speckle tracking LA analysis, as determinants of exercise capacity in patients after MI.
MethodsStudy populationNinety-four consecutive patients were prospectively enrolled one month after an acute MI. Clinical and anthropometric assessment, detailed transthoracic echocardiogram, blood sample collection, and CPET were performed, on the same day, in all patients. Exclusion criteria were age over 75 years, inability to exercise, severe valvular disease, moderate to severe chronic pulmonary disease, anemia (hemoglobin <12 g/l), atrial fibrillation and exercise-induced myocardial ischemia.
Baseline demographic, clinical and echocardiographic variables were acquired and their independent associations with exercise performance parameters were analyzed.
The study protocol conforms to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all patients and the local institutional review board approved the study protocol.
Echocardiographic dataAll echocardiographic studies were acquired by a single experienced operator using an iE33 ultrasound system (Philips Medical Systems, Best, The Netherlands) equipped with an S5-1 transducer. Images were digitally stored for subsequent offline analysis.
Cardiac chamber dimensions and volumes and left ventricular (LV) mass were measured according to current recommendations for cardiac chamber quantification.9 Pulsed-wave Doppler velocities at the upper right pulmonary vein and mitral inflow velocities were assessed according to recommendations for assessment of LV diastolic function.10 Pulsed-wave tissue Doppler velocities – systolic (S’), early diastolic (E’) and late diastolic (A’) – were acquired in apical 4-chamber view, with the sample positioned at the septal and lateral mitral annulus. All velocities were recorded at end-expiration and averaged over three consecutive cardiac cycles.
Systolic function was assessed by calculating LV ejection fraction using the modified Simpson's rule from biplane 4- and 2-chamber views and by analyzing systolic myocardial annular tissue velocity (septal S’, lateral S’ and mean S’). Diastolic function was assessed according to the latest consensus guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI)10 by determining peak early (E) and late (A) diastolic mitral inflow velocities, early LV filling deceleration time, E/A ratio, the septal, lateral and mean myocardial annular tissue velocity (septal E’, lateral E’ and mean E’, respectively), E/E’ ratio (also at the septal, lateral and mean E/E’), pulmonary vein flow analysis by the difference between the duration of the reversed atrial wave of pulmonary venous flow (Ard) and the mitral A-wave duration (Ad) (Ard-Ad), and isovolumic relaxation time. Also on the basis of the ASE/EACVI guidelines on evaluation of diastolic function,10 patients were categorized in terms of diastolic dysfunction (DD) as normal, grade I (mild DD), grade II (moderate DD) or grade III (severe DD) by two independent cardiologists who were blinded to the CPET data. In the event of disagreement, each case was discussed individually, and if doubt persisted no grade was endorsed.
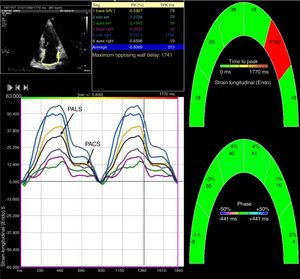
Analysis of left atrial dimensions and functional and deformation parametersTwo-dimensional grayscale images were acquired in apical 4- and 2-chamber views, with a frame rate between 70 and 100 frames/s. Three cardiac cycles were digitally stored. The LA endocardial border was manually traced and analysis was performed using Velocity Vector Imaging (VVI) software (syngo VVI 2.0, Siemens Medical Solutions USA, Inc) by one observer blinded to the clinical data, as previously described.10–12 As shown in Figure 1, the software divides the left atrium into six segments, and tracking quality was visually checked in all segments. Segments that the software failed to track were adjusted manually. Patients with inadequate tracking in more than two segments were excluded from the study. Adequate tracking quality was achieved in 98% of patients (two patients were excluded due to inadequate imaging). Time-volume curves were extracted from the displacement of the LA endocardial pixels from which maximum LA volume (LAVmax), minimum LA volume (LAVmin) and LA volume before atrial contraction (LAV pre-A) were calculated, as previously described.13 All LA volumes were indexed to body surface as recommended.9
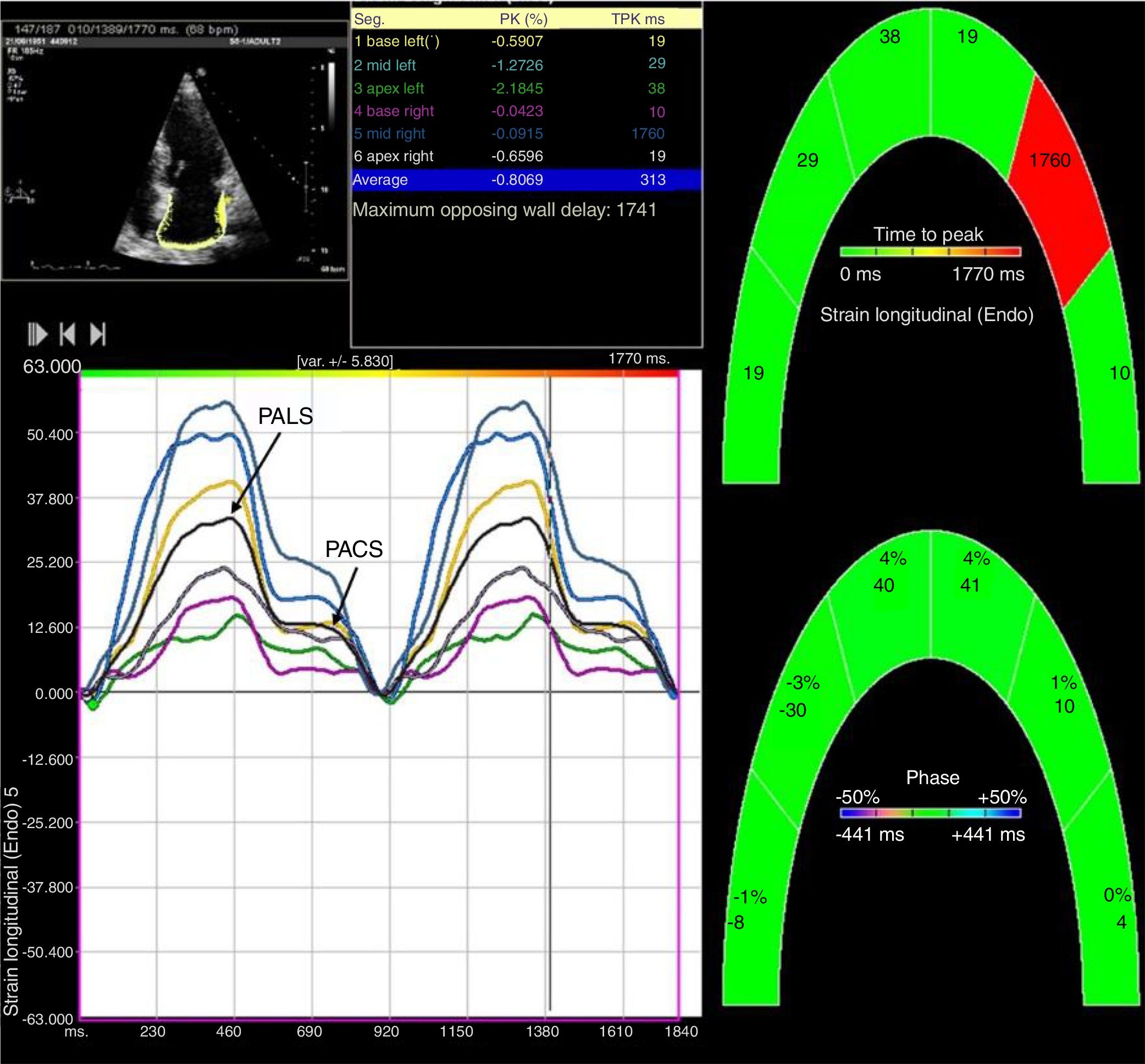
Two-dimensional left atrial speckle tracking analysis for the determination of left atrial (LA) strain: 4-chamber view depicting the corresponding LA strain curves for each of six segments analyzed in each view. Peak atrial longitudinal strain (PALS) is a measure of LA reservoir function and peak atrial contraction strain (PACS) is a marker of LA pump function. Conduit strain was calculated as the difference between PALS and PACS.
LA function was assessed from LA volumes using several validated formulas tested in previous studies.4,12,14 LA reservoir volume (also known as LA stroke volume) was calculated as LAVmax-LAVmin, LA conduit volume as LAVmax-LA pre-A and LA contractile volume as LA pre-A-LAVmin. LA ejection fraction was calculated as (LAVmax-LAVmin)/LAVmax×100. LA booster function, which is an index of LA active contraction, was determined as (LA pre-A-LAVmin)/LA pre-A×100 and LA conduit function, an index of passive function, as (LAVmax-LA pre-A)/LAVmax×100. Finally, LA reservoir function, which is an expansion index, was determined as (LAVmax-LAVmin)/LAVmin×100.
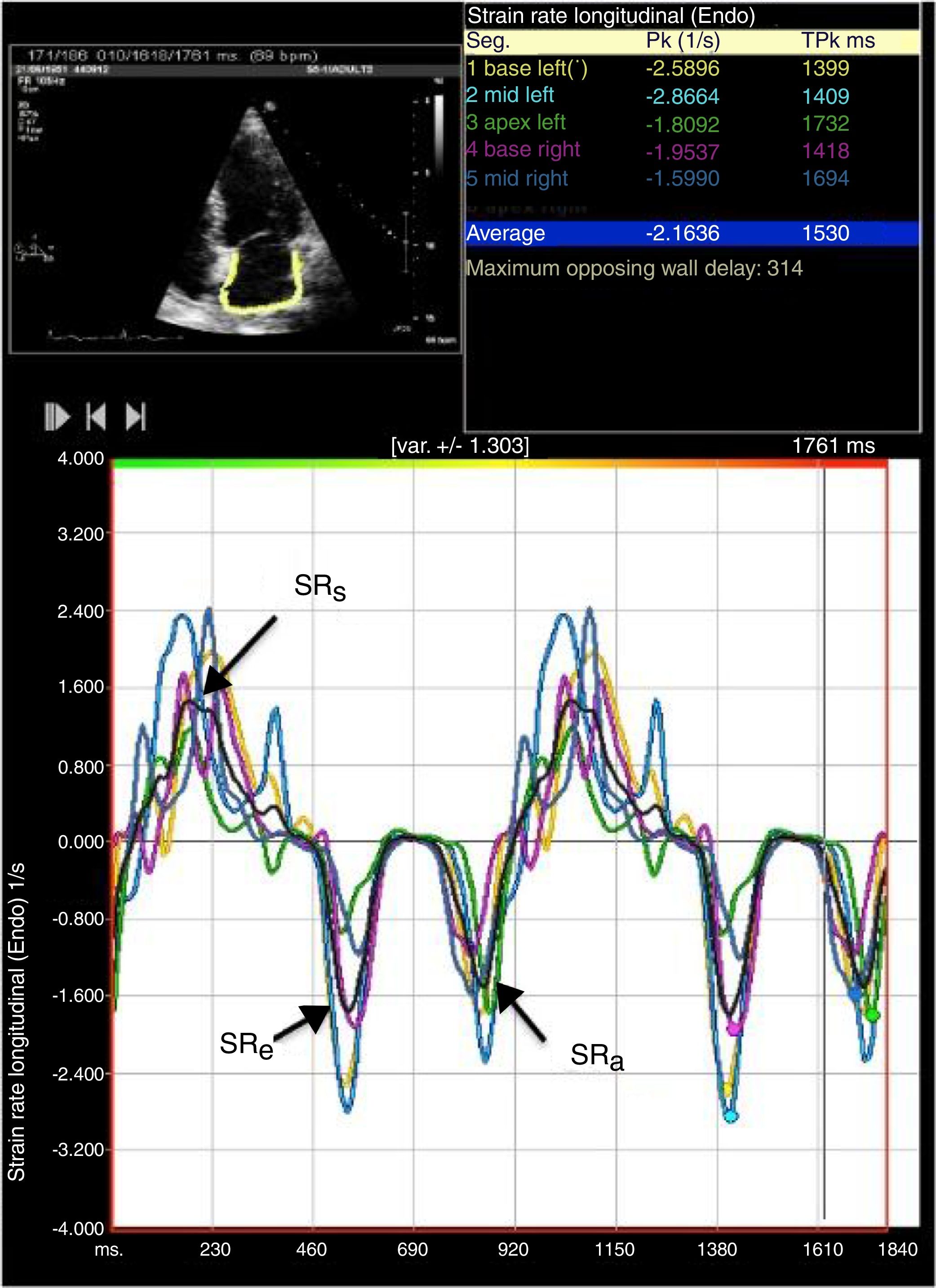
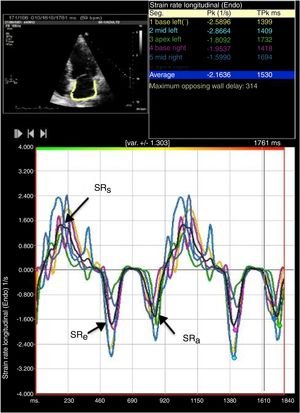
LA strain and strain rate were assessed using the same software, by tracking and comparing the relative position of speckles throughout the cardiac cycle. Strain curves were displayed for each of the six segments automatically generated by the software. Zero strain was set at QRS onset. As shown in Figure 1, using this reference point, the LA strain pattern consists of a positive wave that peaks at the end of ventricular systole, followed by a decrease after the opening of the mitral valve and, after a plateau, by a second decrease that corresponds to atrial contraction. From the average of the strain curves of all segments we calculated peak LA strain at the end of ventricular systole (peak atrial longitudinal strain, PALS), which is a measure of LA reservoir function,12 and peak atrial contraction strain (PACS), which can be considered a marker of LA pump function, as previously described.12,15 Passive emptying (conduit) strain was calculated as the difference between PALS and PACS. Strain rate analysis was used to measure peak LA strain rate during ventricular systole (SRs), during early LV filling (SRe) and during atrial contraction (SRa), as shown in Figure 2.
Two-dimensional left atrial (LA) speckle tracking analysis for the determination of left atrial strain rate: 4-chamber view showing LA strain rate curves for the six segments analyzed, with determination of peak strain rate during systole (SRs), peak strain rate during early diastole (SRe) and peak strain rate during atrial contraction (SRa).
Each patient underwent symptom-limited treadmill CPET using the modified Bruce protocol. Expired gases were collected continuously throughout exercise and analyzed for ventilatory volume and for oxygen (O2) and carbon dioxide (CO2) content using dedicated analyzers. Standard spirometry to determine forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) was performed before the exercise test. Equipment calibration and all measurements were carried out according to the recommendations of the American Thoracic Society and American College of Chest Physicians.16 The following variables were calculated: peak oxygen consumption (pVO2) measured in ml/kg/min; peak respiratory exchange ratio, defined as the ratio of CO2 production to O2 consumption at peak effort; VE/VCO2 slope, defined as the slope of the increase in peak ventilation/increase in CO2 production throughout exercise; anaerobic threshold, defined as the point at which CO2 production increased disproportionately in relation to O2 consumption obtained from a graph plotting O2 consumption against CO2 production; and total exercise duration in s. Patients were not asked to discontinue beta-blockers before the test.
Statistical analysisStatistical analyses were performed using IBM SPSS Statistics, version 20 (IBM SPSS Inc., Chicago, IL, USA). All p-values are two-tailed and a significance level of 5% was used. Data are expressed as mean and standard deviation for quantitative variables with normal distribution or as median (25th-75th percentile) for variables with non-normal distribution. Categorical variables are expressed as number and percentage. Differences between groups were assessed using the Student's t test and Mann-Whitney U test for comparisons of quantitative variables, and chi-square tests for discrete variables. The bivariate correlations between LA parameters and CPET parameters were assessed by Pearson's (r) or Spearman's correlation coefficient (ρ), as appropriate.
Ten patients were randomly selected to test the intra- and interobserver reproducibility of LA measurements. For intraobserver variability, the same operator performed a second measurement, more than a month after the initial analysis. For interobserver variability, a second operator analyzed the same loops. Bland-Altman analyses were performed, and the intraclass correlation coefficient and coefficient of variation were calculated. The software showed good intra- and interobserver agreement, as detailed in previous studies by our group.17
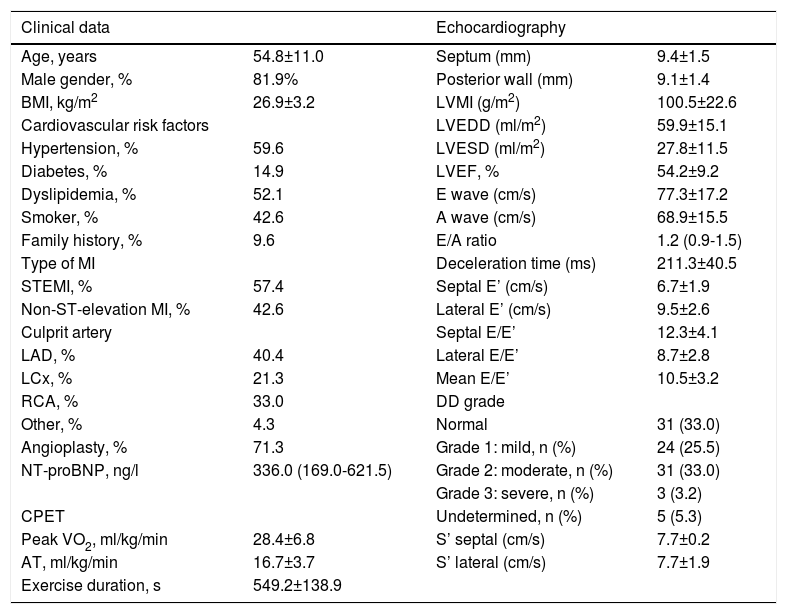
ResultsThe baseline characteristics of the study population are outlined in Table 1. A total of 94 patients were assessed, predominantly male (n=77, 81.9%), with a mean age of 54.8±11.0 years, with both ST- and non-ST-elevation MI (57.4% and 42.6%, respectively). The left anterior descending artery was the culprit artery in 40.4% and the majority underwent percutaneous coronary intervention (71.3%). Patients received optimal medical therapy: dual antiplatelet therapy and a statin in 100%, a renin-angiotensin system inhibitor in 96.8%, a beta-blocker in 95.7% and an aldosterone antagonist in 15.0%. Echocardiographic characteristics one month after MI are also shown in Table 1. Mean ejection fraction was 54.2±9.2% and there was a high prevalence of diastolic dysfunction (61.7%). LA volumes and functional and deformation parameters are displayed in Table 2.
Characteristics of the study population.
| Clinical data | Echocardiography | ||
|---|---|---|---|
| Age, years | 54.8±11.0 | Septum (mm) | 9.4±1.5 |
| Male gender, % | 81.9% | Posterior wall (mm) | 9.1±1.4 |
| BMI, kg/m2 | 26.9±3.2 | LVMI (g/m2) | 100.5±22.6 |
| Cardiovascular risk factors | LVEDD (ml/m2) | 59.9±15.1 | |
| Hypertension, % | 59.6 | LVESD (ml/m2) | 27.8±11.5 |
| Diabetes, % | 14.9 | LVEF, % | 54.2±9.2 |
| Dyslipidemia, % | 52.1 | E wave (cm/s) | 77.3±17.2 |
| Smoker, % | 42.6 | A wave (cm/s) | 68.9±15.5 |
| Family history, % | 9.6 | E/A ratio | 1.2 (0.9-1.5) |
| Type of MI | Deceleration time (ms) | 211.3±40.5 | |
| STEMI, % | 57.4 | Septal E’ (cm/s) | 6.7±1.9 |
| Non-ST-elevation MI, % | 42.6 | Lateral E’ (cm/s) | 9.5±2.6 |
| Culprit artery | Septal E/E’ | 12.3±4.1 | |
| LAD, % | 40.4 | Lateral E/E’ | 8.7±2.8 |
| LCx, % | 21.3 | Mean E/E’ | 10.5±3.2 |
| RCA, % | 33.0 | DD grade | |
| Other, % | 4.3 | Normal | 31 (33.0) |
| Angioplasty, % | 71.3 | Grade 1: mild, n (%) | 24 (25.5) |
| NT-proBNP, ng/l | 336.0 (169.0-621.5) | Grade 2: moderate, n (%) | 31 (33.0) |
| Grade 3: severe, n (%) | 3 (3.2) | ||
| CPET | Undetermined, n (%) | 5 (5.3) | |
| Peak VO2, ml/kg/min | 28.4±6.8 | S’ septal (cm/s) | 7.7±0.2 |
| AT, ml/kg/min | 16.7±3.7 | S’ lateral (cm/s) | 7.7±1.9 |
| Exercise duration, s | 549.2±138.9 |
Data are presented as mean and standard deviation or as median (25th-75th percentile) for continuous variables, and as number and percentage for categorical variables.
AT: anaerobic threshold; BMI: body mass index; CPET: cardiopulmonary exercise testing; DD: diastolic dysfunction; LAD: left anterior descending artery; LCx: left circumflex artery; LVEDD: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic volume; LVMI: left ventricular mass index; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction.
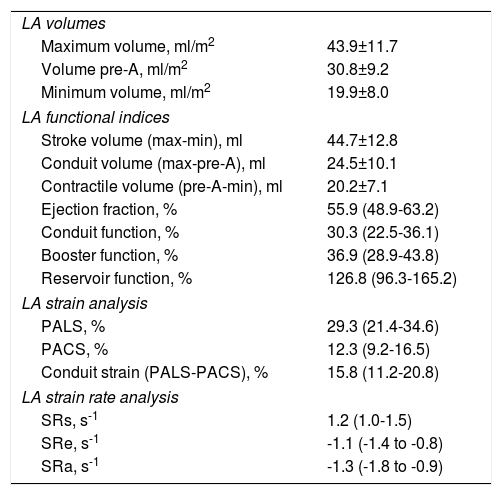
Left atrial size and functional and deformation parameters.
| LA volumes | |
| Maximum volume, ml/m2 | 43.9±11.7 |
| Volume pre-A, ml/m2 | 30.8±9.2 |
| Minimum volume, ml/m2 | 19.9±8.0 |
| LA functional indices | |
| Stroke volume (max-min), ml | 44.7±12.8 |
| Conduit volume (max-pre-A), ml | 24.5±10.1 |
| Contractile volume (pre-A-min), ml | 20.2±7.1 |
| Ejection fraction, % | 55.9 (48.9-63.2) |
| Conduit function, % | 30.3 (22.5-36.1) |
| Booster function, % | 36.9 (28.9-43.8) |
| Reservoir function, % | 126.8 (96.3-165.2) |
| LA strain analysis | |
| PALS, % | 29.3 (21.4-34.6) |
| PACS, % | 12.3 (9.2-16.5) |
| Conduit strain (PALS-PACS), % | 15.8 (11.2-20.8) |
| LA strain rate analysis | |
| SRs, s-1 | 1.2 (1.0-1.5) |
| SRe, s-1 | -1.1 (-1.4 to -0.8) |
| SRa, s-1 | -1.3 (-1.8 to -0.9) |
LA: left atrial; PACS: peak atrial contraction strain; PALS: peak atrial longitudinal strain; pre-A: volume immediately before atrial contraction; SRa: strain rate during atrial contraction; SRe: strain rate during early diastole; SRs: strain rate during systole.
A significant association was found between VO2/kg and classical determinants of exercise capacity, namely age (r=-0.41; p<0.001), body mass index (r=-0.27; p=0.01) and N-terminal pro-brain natriuretic peptide (NT-proBNP) (ρ=-0.42; p<0.001). Lower VO2/kg was also associated with a previous history of hypertension (p=0.02) and female gender (p<0.001). There was a significant correlation between VO2/kg and diastolic function parameters, particularly septal E’ (r=0.36; p=0.001), lateral E’ (r=0.37; p<0.001), septal E/E’ (r=-0.42; p<0.001), lateral E/E’ (r=-0.33; p<0.01) and mean E/E’ (r=-0.42; p<0.001). Patients with diastolic dysfunction had significantly lower VO2/kg (25.7±7.0 ml/kg/min vs. 29.7±7.4 ml/kg/min in patients with normal diastolic function; p=0.02).
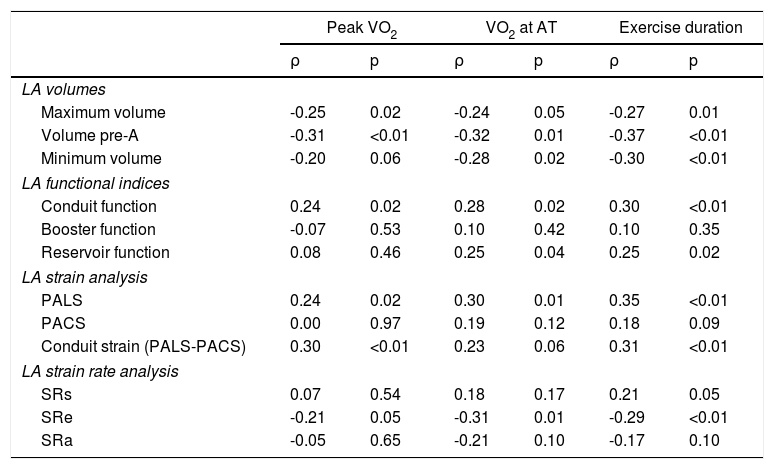
Association between left atrial volumes and functional parameters and exercise capacity
As shown in Table 3, increased LA volumes were correlated with reduced exercise capacity, particularly with reduced peak VO2, reduced VO2 at anaerobic threshold and decreased exercise duration. Among volume parameters, LA pre-A showed the best correlation with exercise capacity.
Correlations between left atrial volumes and functional and deformation parameters and exercise capacity.
| Peak VO2 | VO2 at AT | Exercise duration | ||||
|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | |
| LA volumes | ||||||
| Maximum volume | -0.25 | 0.02 | -0.24 | 0.05 | -0.27 | 0.01 |
| Volume pre-A | -0.31 | <0.01 | -0.32 | 0.01 | -0.37 | <0.01 |
| Minimum volume | -0.20 | 0.06 | -0.28 | 0.02 | -0.30 | <0.01 |
| LA functional indices | ||||||
| Conduit function | 0.24 | 0.02 | 0.28 | 0.02 | 0.30 | <0.01 |
| Booster function | -0.07 | 0.53 | 0.10 | 0.42 | 0.10 | 0.35 |
| Reservoir function | 0.08 | 0.46 | 0.25 | 0.04 | 0.25 | 0.02 |
| LA strain analysis | ||||||
| PALS | 0.24 | 0.02 | 0.30 | 0.01 | 0.35 | <0.01 |
| PACS | 0.00 | 0.97 | 0.19 | 0.12 | 0.18 | 0.09 |
| Conduit strain (PALS-PACS) | 0.30 | <0.01 | 0.23 | 0.06 | 0.31 | <0.01 |
| LA strain rate analysis | ||||||
| SRs | 0.07 | 0.54 | 0.18 | 0.17 | 0.21 | 0.05 |
| SRe | -0.21 | 0.05 | -0.31 | 0.01 | -0.29 | <0.01 |
| SRa | -0.05 | 0.65 | -0.21 | 0.10 | -0.17 | 0.10 |
ρ: Spearman's correlation coefficient; AT: anaerobic threshold; LA: left atrial; PACS: peak atrial contraction strain; PALS: peak atrial longitudinal strain; pre-A: volume immediately before atrial contraction; SRa: strain rate during atrial contraction; SRe: strain rate during early diastole; SRs: strain rate during systole.
A positive correlation was found between LA conduit function and exercise capacity (Table 3). By contrast, LA booster function, an index of LA active contraction, did not correlate with any exercise performance parameter.
Association between left atrial strain and strain rate and exercise capacityThere was a significant positive correlation between PALS and peak VO2, VO2 at anaerobic threshold, and exercise duration (Table 3). Passive emptying strain (the difference between PALS and PACS) was also significantly correlated with exercise capacity, but no association was found between PACS and exercise capacity.
An inverse correlation was found between exercise capacity parameters and LA strain rate at early diastole, but not with LA strain rate during ventricular systole or atrial contraction.
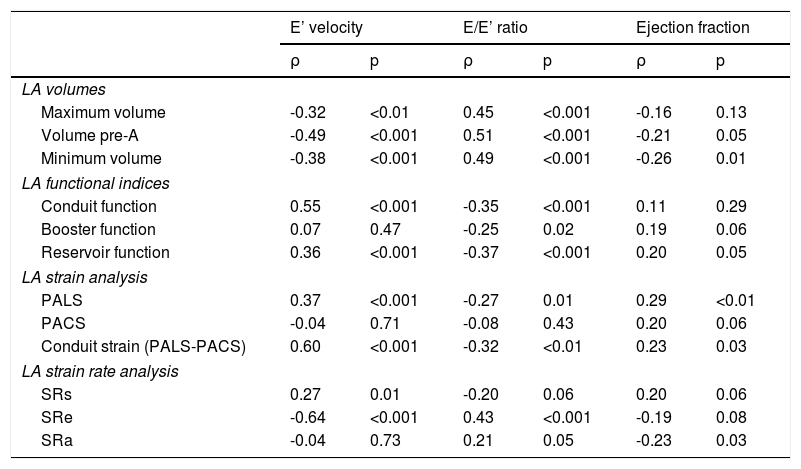
Association between left atrial size and function and left ventricular diastolic and systolic functionAs shown in Table 4, worse LV diastolic function, assessed by E’ velocity and E/E’ ratio, was associated with increased LA volumes, especially with LA volume before atrial contraction. We also observed a direct and significant correlation between E’ velocity and LA conduit function and LA reservoir function, but not with booster function. LA deformation parameters, particularly PALS, PALS-PACS and SRe, showed a strong correlation with E’ velocity and E/E’ ratio. There was a modest correlation between PALS and SRs and ejection fraction.
Correlations (r) between left atrial parameters and diastolic and systolic function.
| E’ velocity | E/E’ ratio | Ejection fraction | ||||
|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | |
| LA volumes | ||||||
| Maximum volume | -0.32 | <0.01 | 0.45 | <0.001 | -0.16 | 0.13 |
| Volume pre-A | -0.49 | <0.001 | 0.51 | <0.001 | -0.21 | 0.05 |
| Minimum volume | -0.38 | <0.001 | 0.49 | <0.001 | -0.26 | 0.01 |
| LA functional indices | ||||||
| Conduit function | 0.55 | <0.001 | -0.35 | <0.001 | 0.11 | 0.29 |
| Booster function | 0.07 | 0.47 | -0.25 | 0.02 | 0.19 | 0.06 |
| Reservoir function | 0.36 | <0.001 | -0.37 | <0.001 | 0.20 | 0.05 |
| LA strain analysis | ||||||
| PALS | 0.37 | <0.001 | -0.27 | 0.01 | 0.29 | <0.01 |
| PACS | -0.04 | 0.71 | -0.08 | 0.43 | 0.20 | 0.06 |
| Conduit strain (PALS-PACS) | 0.60 | <0.001 | -0.32 | <0.01 | 0.23 | 0.03 |
| LA strain rate analysis | ||||||
| SRs | 0.27 | 0.01 | -0.20 | 0.06 | 0.20 | 0.06 |
| SRe | -0.64 | <0.001 | 0.43 | <0.001 | -0.19 | 0.08 |
| SRa | -0.04 | 0.73 | 0.21 | 0.05 | -0.23 | 0.03 |
ρ: Spearman's correlation coefficient; LA: left atrial; PACS: peak atrial contraction strain; PALS: peak atrial longitudinal strain; SRa: strain rate during atrial contraction; SRe: strain rate during early diastole; SRs: strain rate during systole.
In this study we have shown that increased LA volumes are associated with decreased functional capacity in patients after MI. We found a significant correlation between exercise capacity and LA conduit function, but not with contractile function. Lower PALS and reduced SRe correlated with worse exercise capacity parameters, suggesting that LA longitudinal strain analysis may also be useful to predict reduced exercise capacity.
The role of left atrial assessment in cardiovascular diseaseSeveral studies have shown that LA enlargement is a strong predictor of cardiovascular outcomes after MI2,3 and in heart failure18 and atrial fibrillation.19 Beyond their prognostic value, in this study we observed that increased LA volumes, especially LA volume before atrial contraction, were also associated with reduced exercise capacity. This observation is in agreement with another study that shows the same association between poor exercise performance and increased maximum LA volume in patients with heart failure with preserved ejection fraction (HFPEF).20 Furthermore, Galrinho et al. showed there was an inverse correlation between LA volume and VO2 max and that LA enlargement was associated with a higher rate of cardiac events in patients with dilated cardiomyopathy.21
The left atrium plays an important role in the regulation of global cardiac function. It acts as a reservoir during systole, as a conduit during early diastole, and as an active blood pump during late diastole. Recent studies have shown that LA size, as well as reduced LA function, is associated with adverse outcome. In patients after MI, LA ejection fraction was an independent predictor of mortality, providing additional prognostic value to that of maximum LA volume.22 In stable coronary heart disease patients with LV ejection fraction ≥50%, one study related decrease in LA functional indices with higher rates of hospitalization for heart failure.23 Few data are available on the role of LA function in exercise capacity. In two small studies, LA function, assessed only by LA ejection fraction, was associated with reduced exercise capacity in patients with HFPEF24 and with dilated cardiomyopathy.25 In our study, we assessed LA function using several parameters and indices that allow a more complete analysis of the different phases of LA function. We found that decreased exercise capacity was associated with reduced LA conduit function4 but not with LA booster function, which is an index of LA active contraction.4 Finally, we observed that LA reservoir function was a less important marker of reduced functional capacity, because LA expansion index was not significantly related to peak VO2, but showed a modest association with VO2 at anaerobic threshold and reduced exercise duration. However, analysis of parameters derived from strain analysis, especially PALS, may be more accurate for assessing LA reservoir function.
Assessment of left atrial function by strain and strain rate using two-dimensional speckle trackingTwo-dimensional speckle tracking is a relatively new echocardiographic tool that tracks the pattern of myocardial speckles, frame by frame, to calculate strain and strain rate. Strain represents the extent of myocardial deformation, while strain rate represents the speed at which deformation occurs.26 Several studies have demonstrated good feasibility and reproducibility of LA strain analysis by speckle tracking.5,7,8 Interestingly, Cameli et al.27 have demonstrated that LA strain analysis is a predictor of cardiovascular events, showing that LA strain may be more sensitive for the assessment of LA function. Longitudinal studies have also suggested that LA myocardial deformation parameters are reduced before atrial volume increases.28
In our study, LA longitudinal strain parameters such as PALS correlated well with reduced exercise capacity. This observation is consistent with another recent study in patients referred for exercise echocardiography that showed a strong correlation between total LA longitudinal strain and maximal exercise tolerance, assessed only by estimated metabolic equivalents.15 PALS reflects the passive stretching of the left atrium during LV systole and is considered a measure of LA reservoir function.5,12,28 LA reservoir function may be particularly important after MI because it can withstand the impact of increased LA pressure due to LV dysfunction, helping to maintain adequate LV filling. However, in the long term, the sustained increase in LA pressure will induce LA dysfunction and dilatation. It has been shown that reduced PALS after MI is an independent predictor of LA remodeling and reduced LA function at one-year follow-up.28
We found no significant relation between exercise capacity and LA contraction strain, a marker of LA pump function.12,15 Consistent with this finding, we also observed no association between exercise parameters and other measures of LA contraction function, such as LA booster function or LA strain rate during atrial contraction. This reinforces the observation that LA pump function is not a particularly significant determinant of functional capacity, even after MI. Indeed, in atrial fibrillation, although peak VO2 is reduced, the degree of reduction is associated with the underlying cardiac pathologic conditions and not with atrial fibrillation itself.29 Also, in these patients, cardioversion to sinus rhythm only increases exercise capacity by about 5%.30
Interaction between left atrial and left ventricular diastolic function: the role of atrioventricular couplingWith its reservoir, conduit and active contraction phases, the left atrium actively modulates LV function.31 The LA reservoir phase is important for LV filling because the energy stored in the atrium during ventricular systole is released after mitral valve opening, contributing to early diastolic function, and LA reservoir function is impaired in several cardiac diseases that are associated with impaired cardiac filling.28,32 Besides, the LA conduit phase spans early LV filling and diastasis and the LA contractile phase depends on preload, afterload, intrinsic LA contractility and electromechanical coupling. To maintain LV filling at an optimum level during exercise, the LA reservoir, conduit, and pump functions should work in harmony. It is therefore clear that the interaction between LA and LV function – atrioventricular coupling – can directly influence global cardiac function, cardiac output and exercise capacity.15 In a study by Kusunose et al., analyzing patients with preserved LV ejection fraction referred for exercise echocardiography, the association between reduced LA function and impaired exercise capacity was similar to that of elevated LV filling pressures (E/E’ ratio), emphasizing the importance of correct atrioventricular coupling.15
On the other hand, since the left atrium is directly exposed to LV diastolic pressure, LV diastolic dysfunction can lead to an increase in LA size and to changes in the different phases of LA function. LA volume is known to be a sensitive biomarker of the level and duration of LV filling pressures and an indicator of chronic LV diastolic dysfunction.33,34 In our study we also observed a significant correlation between diastolic function parameters (such as E’ velocity and E/E’ ratio) and LA volumes. This observation may explain the association seen between LA volumes and exercise capacity. Interestingly, we found that increased LA volume before atrial contraction, which is more dependent on early LV diastolic function and on LA conduit function,35 was the LA volume parameter that correlated best with exercise parameters. In the PARAMOUNT trial, impairment was found in all phases of LA function (reservoir, conduit and booster functions) in HFPEF patients, however after adjustment for potential confounders, only reservoir function (measured by systolic LA strain) remained significantly reduced.36 We also observed a strong correlation between LV diastolic parameters and LA conduit function and LA reservoir function, but not with LA booster function. Moreover, as another example of the importance of atrioventricular coupling, recent data have shown that different degrees of diastolic dysfunction can influence the different phases of LA function.35,37 In patients with mild diastolic dysfunction, LA conduit function is reduced, which is compensated by an increase in booster function but, as the severity of diastolic dysfunction increases, this mechanism no longer operates, resulting in a reduction of LV filling volume.37
LA strain and strain rate parameters assessed by speckle tracking echocardiography are also significantly correlated with LV diastolic function.6,12,38,39 Our data are consistent with these observations, showing a significant correlation between E’ velocity and E/E’ ratio, and peak LA systolic strain and LA conduit strain. As expected, we also found a significant association between diastolic function and LA strain rate, especially LA strain rate during early diastole.
Finally, regarding the association between LA functional parameters and systolic function, the available data are conflicting.12,38 However, in a study by Wakami et al., in which invasive measurements were made in patients undergoing cardiac catheterization, LA systolic strain was mainly correlated with LV end-diastolic pressures but also with LV ejection fraction.38 Furthermore, Sanchis et al. related impairment of LA reservoir and contractile function (assessed by LA peak systolic strain rate and LA peak strain rate after contraction, respectively) with both HFPEF and heart failure with reduced ejection fraction.39 In our study we also found a modest but significant association between LA reservoir function (assessed by both LA peak systolic strain and LA expansion index) and ejection fraction.
Study strengths and limitationsThe present study extends previous results that emphasize the clinical relevance of analyzing LA size and function in various clinical settings. Beyond the classical parameters, we also assessed LA function by novel speckle-tracking imaging parameters. In this study, exercise capacity was assessed using data on VO2 from CPET, which is the most accurate, reliable and reproducible measurement of exercise tolerance.1 Exercise capacity using peak VO2 is considered the gold standard parameter for assessment of functional capacity.16 We also assessed submaximal exercise capacity by determining the ventilatory anaerobic threshold, using the V-slope method, which has the advantage of being relatively effort-independent.1
In this study, LA function was assessed at rest. Measurements obtained during exercise might have been more predictive of exercise intolerance. It is likely that previous diastolic dysfunction could influence LA function and be partially responsible for the association between LA function and decreased exercise capacity. Although strain by speckle tracking has been widely used for LA assessment,5,7,8,28 and appears to predict cardiovascular outcomes,27 no software has been validated specifically for the assessment of LA strain. Finally, given the inter-vendor variability in strain measurements, these results can only be extrapolated for the software used in this study.
ConclusionBeyond their prognostic value, increased LA volumes can be useful to predict reduced exercise capacity in patients after MI. In our study, reduced exercise capacity was associated with reduced LA conduit function, but not with LA contractile function. Lower PALS and reduced SRe also correlated with worse exercise parameters. LA size and functional parameters were interdependent with LV diastolic function, highlighting the importance of correct atrioventricular coupling in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the Portuguese Foundation for Science and Technology grants PEst-C/SAU/UI0051/2011 and EXCL/BIM-MEC/0055/2012 through the Cardiovascular R&D Unit and by European Commission grant FP7-Health-2010; MEDIA-261409.