Late gadolinium enhancement (LGE) extent has emerged as a predictor of sudden cardiac death (SCD) in patients with hypertrophic cardiomyopathy (HCM), however little is known about the arrhythmogenic relevance of its specific location in the left ventricle. Our aim was to analyze the influence of LGE location on the occurrence of ventricular arrhythmias (VA) and SCD in patients with HCM.
MethodsWe performed a retrospective analysis of clinical and Holter records of HCM patients who underwent cardiac magnetic resonance at our center. LGE extent and distribution were assessed using the American Heart Association 17-segment model. VA was defined as non-sustained or sustained ventricular tachycardia, ventricular fibrillation or SCD.
ResultsSixty-one patients (age 57.0±16.7 years) were included and VA occurred in 24.6% (n=15). Patients with VA showed greater LGE extent than those without (7.40±5.3 vs. 3.52±3.0 segments, p=0.007). Analyzing the distribution of LGE, a set of arrhythmogenic segments (apex/basal inferior/basal anterolateral/mid inferoseptal) was found. The extent of LGE involvement in these segments was also greater in patients with VA (2.07±1.03 vs. 0.65±0.71 segments, p<0.001; area under the curve 0.861 for VA) and this difference remained significant after adjustment for potentially confounding variables.
ConclusionsThe extent of LGE involvement of a set of segments with an apparent relation to cardiac areas of increased mechanical stress was significantly and independently associated with the occurrence of VA, suggesting that not only the extent but also the location of LGE is important for the assessment of SCD risk in HCM patients.
A extensão do realce tardio (RT) tem surgido como preditor de morte súbita cardíaca (MSC) em doentes com miocardiopatia hipertrófica (MCH), contudo pouco se conhece quanto à relevância arritmogénica da sua localização específica no ventrículo esquerdo. O nosso objetivo foi avaliar a influência da localização do RT na ocorrência de arritmias ventriculares/morte súbita (AV) em doentes com MCH.
MétodosAnálise retrospetiva de dados clínicos e Holters de doentes com MCH submetidos a ressonância magnética no nosso centro. A extensão e a distribuição do RT foram avaliadas usando o modelo de 17 segmentos da American Heart Association. Foi definido AV como a ocorrência de taquicardia ventricular não sustentada ou sustentada, fibrilhação ventricular ou paragem cardíaca súbita.
ResultadosForam incluídos 61 doentes (57,0±16,7 anos), tendo ocorrido AV em 24,6% (n=15). Os doentes com AV apresentavam maior extensão de RT do que aqueles sem AV (7,40±5,3 versus 3,52±3,0 segmentos, p=0,007). Analisando a distribuição do RT foi encontrado um conjunto de segmentos arritmogénicos (basal inferior/basal anterolateral/médio inferosseptal/ápex). A extensão de RT nestes segmentos foi também maior nos doentes com AV (2,07±1,03 versus 0,65±0,71 segmentos, p<0,001; área sob a curva ROC 0,861 para AV). Esta diferença permaneceu significativa após ajuste para potenciais variáveis confundidoras.
ConclusõesA extensão do RT num conjunto de segmentos com aparente relação com áreas cardíacas de maior stress mecânico associou-se significativa e independentemente à ocorrência de AV, sugerindo que não apenas a extensão mas também a localização do RT é importante na avaliação do risco de MSC em doentes com MCH.
Hypertrophic cardiomyopathy (HCM) is a common genetic disease, affecting approximately 1 in 500 individuals.1 HCM is histologically characterized by disorganized hypertrophied myocytes and varying amounts of interstitial fibrosis. In addition, structural abnormalities in subendocardial arterioles cause small vessel ischemia, leading to cell death and replacement fibrosis.2 The electrophysiological mechanisms underlying sudden cardiac death (SCD) in HCM patients are not completely understood. The dominant hypothesis postulates that the architectural disorganization of this disease promotes the occurrence of re-entry ventricular arrhythmias (VA), which in turn lead to SCD.2,3
Despite the advances achieved,4 SCD risk stratification in HCM remains imperfect, so there is considerable interest in new risk markers. Late gadolinium enhancement (LGE) assessed by cardiac magnetic resonance (CMR) is used as an imaging surrogate for replacement fibrosis. Given its high prevalence in HCM (55-70%), its use as a dichotomous variable is a poor predictor for SCD, but LGE extent quantified as a percentage of left ventricular (LV) mass was found to be an independent risk factor.5–7
The specific location of LGE in the left ventricle is associated with outcomes in conditions including myocarditis8 and mitral valve prolapse.9 In a large cohort of dilated cardiomyopathy patients the combined presence of septal and free-wall LGE was associated with SCD.10 In the same study, it was found that beyond small amounts, LGE extent had little incremental value in predicting outcomes; predictive models using LGE presence and location were superior to those using LGE extent or pattern. In HCM, LGE located at the right ventricular insertion points has been shown not to correlate with SCD.11,12 However, little is known about its arrhythmogenic relevance in specific LV locations in HCM.
ObjectivesWe hypothesised that some areas of the myocardium could have a higher arrhythmogenic potential if scarred. The aim of our study was to analyze the influence of LGE location on the occurrence of VA and sudden death in patients with HCM.
MethodsStudy population and clinical and arrhythmia parametersWe performed a retrospective analysis of clinical and Holter monitoring records of HCM patients who underwent CMR at our center between July 2009 and November 2016. Patients younger than 16 years old and those with HCM associated with metabolic, infiltrative or syndromic diseases; with evidence of coronary artery disease (defined as known ≥50% coronary artery stenosis or presence of subendocardial LGE on CMR), congenital heart disease or primary valvular heart disease; with subepicardial fibrosis; and those without a Holter monitoring record were excluded. VA was defined as non-sustained ventricular tachycardia (≥3 consecutive ventricular beats at ≥120 bpm and <30 s in duration), sustained ventricular tachycardia, ventricular fibrillation or SCD. Sudden death cases were identified using clinical records and the national health data platform. Five-year SCD risk was estimated using the score proposed in the European guidelines.4
HCM patients were offered genetic testing according to the attending physician's decision and after patient consent. All were tested for the presence or absence of mutations in the following sarcomere protein genes: MYH7, MYBPC3, TNNT2, TNNI3, MYL2, MYL3, ACTC1, TPM1 and CSRP3.
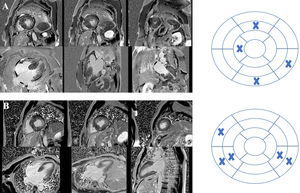
Cardiac magnetic resonance acquisition and analysisCMR imaging was performed on a 1.5-T scanner (Siemens MAGNETOM Symphony TIM, Siemens, Germany). Cine images for functional analyses were acquired using a steady-state free precession sequence during breath-hold and with retrospective ECG triggering. LGE was assessed 10-15 min after intravenous administration of 0.2 mmol/kg of gadobutrol (Gadavist®, Bayer HealthCare Pharmaceuticals, Germany) using a phase-sensitive inversion recovery sequence. The presence and distribution of LGE were qualitatively assessed by two expert readers using the American Heart Association 17-segment model (Figure 1). LGE extent was quantified by visual assessment of the number of myocardial segments involved. Mitral valve systolic anterior motion and left ventricular outflow tract (LVOT) gradient were classified as present or absent.
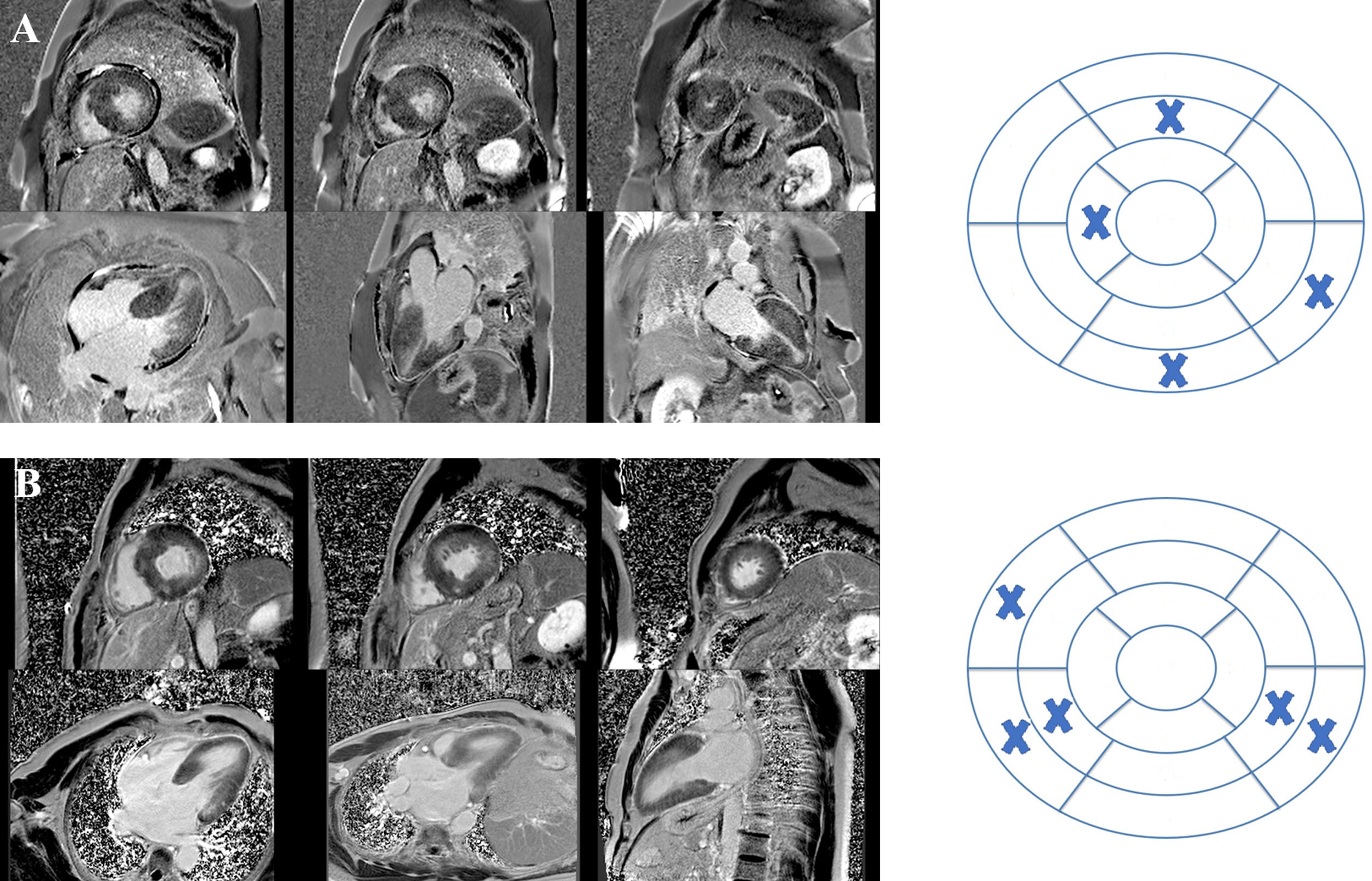
Cardiac magnetic resonance imaging of two patients with hypertrophic cardiomyopathy included in the study showing late gadolinium enhancement (LGE) and the respective bull's eyes marking location and extent of LGE. (A) Patient with a pathogenic mutation in TNNI3; LGE was documented in four segments – basal inferior, basal inferolateral, mid anterior and distal septal; (B) patient with no pathogenic mutation found on genetic testing; LGE was documented in five segments – basal and mid inferoseptal, basal and mid inferolateral, and basal anteroseptal.
Comparisons between groups were performed using the t test, the Mann-Whitney U test, the chi-square test or Fisher's exact test, as appropriate. Correlations were measured by Spearman's coefficient. All reported p-values are two-sided. Statistical significance was defined as a p-value <0.05. Multivariate logistic regression was performed to adjust for possible confounders. All statistical analyses were carried out with IBM® SPSS Statistics® version 22.
ResultsA total of 61 HCM patients (62.3% male, mean age 57.0±16.7 years) were included in the study; mean follow-up was 44.7±24.4 months. Genetic testing was performed in 45.9% of patients (n=28) and a pathogenic or likely pathogenic mutation was found in 53.6% of those tested (n=15). MYBPC3 (n=6), MYH7 (n=4) and TNNI3 (n=3) were the most frequently affected genes. VA occurred in 24.6% of patients (n=15): non-sustained ventricular tachycardia in 13 patients and sudden death in two.
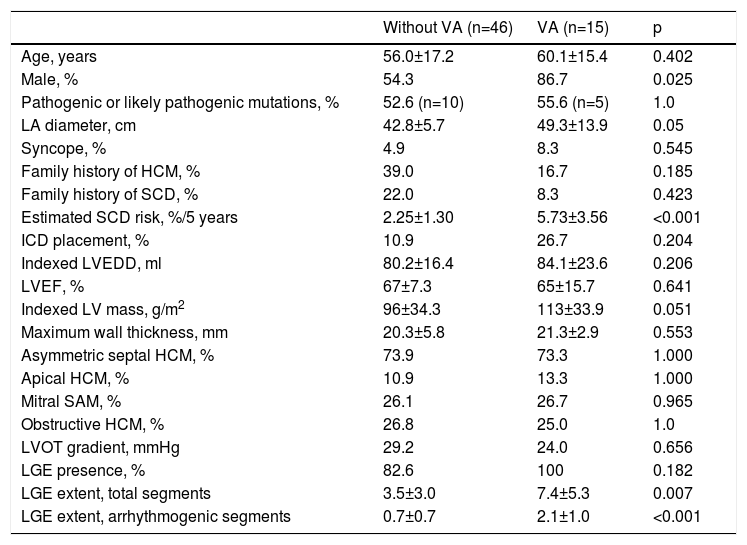
Table 1 summarizes clinical and structural characteristics stratified for VA occurrence. Comparing the two groups there were no differences in mean age, maximum LV wall thickness or indexed LV mass. The presence of a pathogenic or likely pathogenic mutation was also not associated with the occurrence of VA. However, patients with VA were more frequently male (86.7% vs. 54.3%, p=0.025) and had greater LGE extent at CMR (7.40±5.3 vs. 3.52±3.0 segments, p=0.007).
Clinical and structural characteristics of hypertrophic cardiomyopathy patients with and without ventricular arrhythmias.
| Without VA (n=46) | VA (n=15) | p | |
|---|---|---|---|
| Age, years | 56.0±17.2 | 60.1±15.4 | 0.402 |
| Male, % | 54.3 | 86.7 | 0.025 |
| Pathogenic or likely pathogenic mutations, % | 52.6 (n=10) | 55.6 (n=5) | 1.0 |
| LA diameter, cm | 42.8±5.7 | 49.3±13.9 | 0.05 |
| Syncope, % | 4.9 | 8.3 | 0.545 |
| Family history of HCM, % | 39.0 | 16.7 | 0.185 |
| Family history of SCD, % | 22.0 | 8.3 | 0.423 |
| Estimated SCD risk, %/5 years | 2.25±1.30 | 5.73±3.56 | <0.001 |
| ICD placement, % | 10.9 | 26.7 | 0.204 |
| Indexed LVEDD, ml | 80.2±16.4 | 84.1±23.6 | 0.206 |
| LVEF, % | 67±7.3 | 65±15.7 | 0.641 |
| Indexed LV mass, g/m2 | 96±34.3 | 113±33.9 | 0.051 |
| Maximum wall thickness, mm | 20.3±5.8 | 21.3±2.9 | 0.553 |
| Asymmetric septal HCM, % | 73.9 | 73.3 | 1.000 |
| Apical HCM, % | 10.9 | 13.3 | 1.000 |
| Mitral SAM, % | 26.1 | 26.7 | 0.965 |
| Obstructive HCM, % | 26.8 | 25.0 | 1.0 |
| LVOT gradient, mmHg | 29.2 | 24.0 | 0.656 |
| LGE presence, % | 82.6 | 100 | 0.182 |
| LGE extent, total segments | 3.5±3.0 | 7.4±5.3 | 0.007 |
| LGE extent, arrhythmogenic segments | 0.7±0.7 | 2.1±1.0 | <0.001 |
Data are presented as mean ± standard deviation or percentage, unless otherwise indicated.
HCM: hypertrophic cardiomyopathy; ICD: implantable cardioverter-defibrillator; LA: left atrial; LGE: late gadolinium enhancement; LV: left ventricular; LVEDD: end-diastolic volume; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; SAM: systolic anterior motion; SCD: sudden cardiac death; VA: ventricular arrhythmia/sudden death.
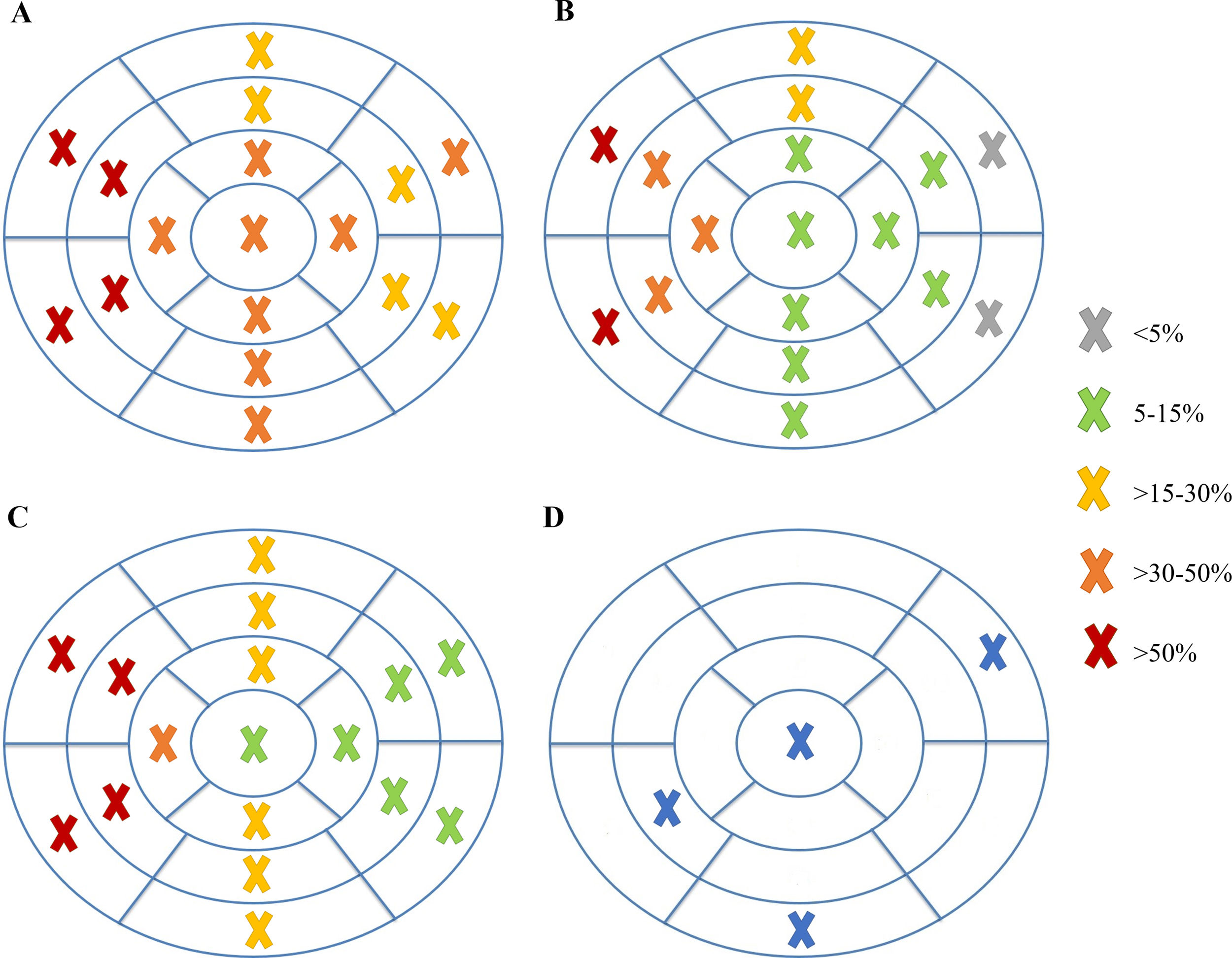
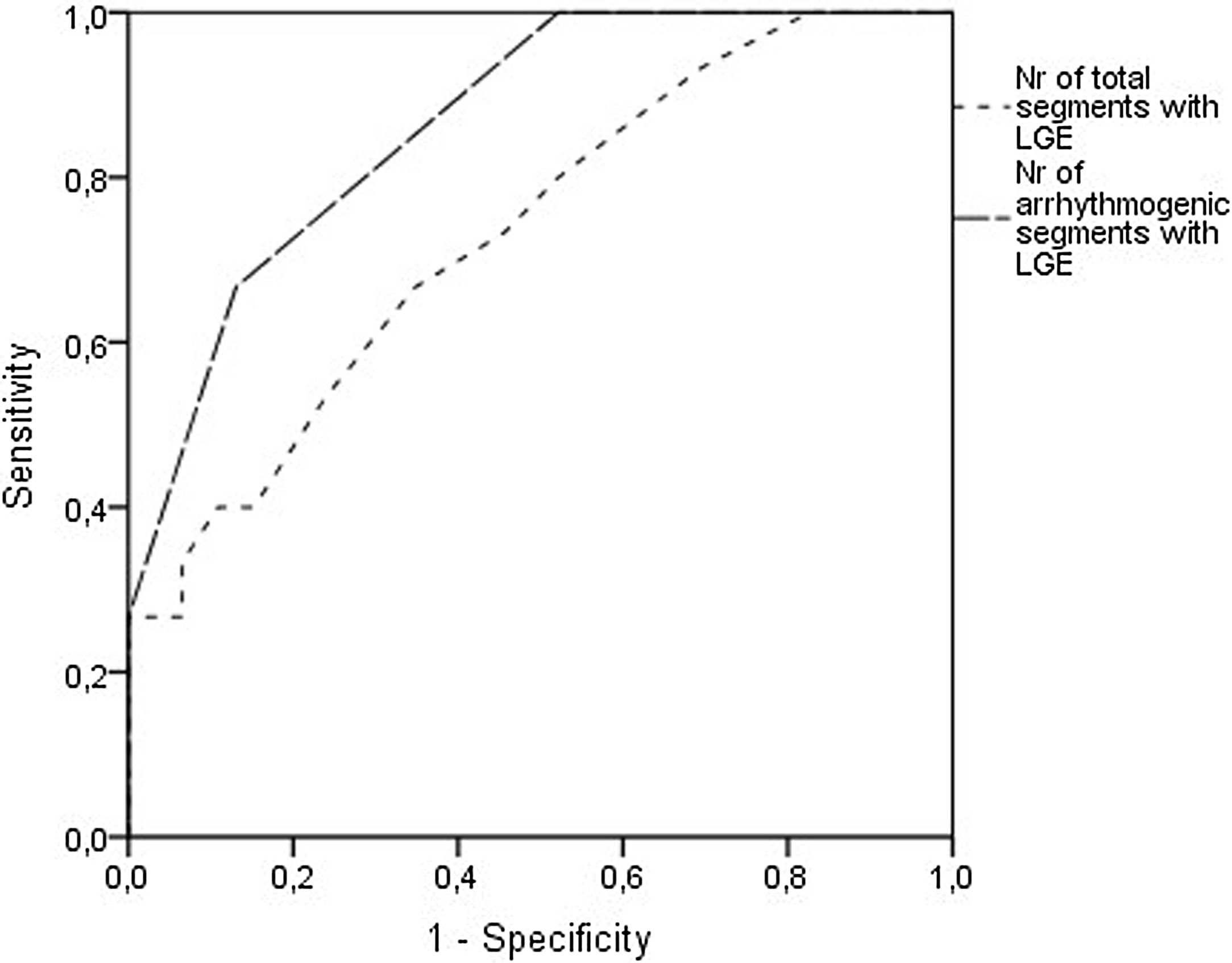
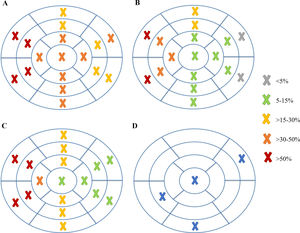
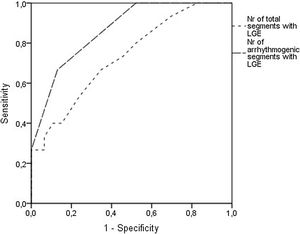
Analyzing each of the 17 myocardial segments individually (Figure 2), septal segments were the most frequently involved by LGE in both groups, but compared to patients without VA, patients with VA had more frequent involvement of apical (40% vs. 6.5%, p=0.005), basal inferior (46.7% vs. 8.7%, p=0.003), basal inferolateral (26.7% vs. 4.3%, p=0.028), basal anterolateral (40% vs. 4.3%, p=0.002), mid inferoseptal (80% vs. 46.7%, p=0.02) and apical lateral (33.3% vs. 6.5%, p=0.017) segments. In multivariate analysis including these six segments, LGE involvement of the basal inferior, basal anterolateral, mid inferoseptal and apical segments (hereinafter referred to as arrhythmogenic segments) remained independently associated with the occurrence of VA. In patients with VA the number of arrhythmogenic segments involved by LGE was significantly higher than in those without (2.1±1.0 vs. 0.7±0.7 segments, p<0.001). Furthermore, none of the 22 patients with no LGE in arrhythmogenic segments developed VA. The area under the receiver operating characteristic curve for predicting VA using LGE extent in arrhythmogenic segments was 0.865 (95% confidence interval 0.769-0.962), which was higher than that using the total number of segments involved by LGE (Figure 3).
Mean estimated five-year SCD risk was 3.05±2.49% (76.9% patients stratified as low risk and 9.6% as high risk). No significant correlation was observed between estimated SCD risk and the total number of segments involved by LGE (r=0.26; p=0.058), however a significant correlation between estimated SCD risk and LGE extent in arrhythmogenic segments was observed (r=0.45, p=0.001).
In multivariate analysis, the extent of LGE involvement in arrhythmogenic segments remained a predictor of VA even after adjustment for the total number of segments involved by LGE, gender, left atrial diameter and other known risk factors for SCD (age, maximum LV wall thickness, syncope, family history of SCD and peak LVOT gradient) (p=0.003).
Analyzing only patients with asymmetric septal hypertrophic cardiomyopathy (n=45), the number of arrhythmogenic segments involved was also higher in patients with VA than in those without (2.0±1.0 vs. 0.6±0.6, p<0.001) and remained a predictor of the primary outcome even after adjustment for the total number of segments involved by LGE.
DiscussionLGE extent is increasingly recognized as an SCD risk predictor in patients with HCM.5–7 However, little is known about the arrhythmogenic relevance of its specific location in different myocardial segments. A previous study in HCM including only patients with LGE aimed to determine a relationship between LGE extent and location and the occurrence of ventricular arrhythmias. LGE extent was assessed using the 17-segment model by counting the number of segments affected; location was divided into septal and non-septal. It was found that the total number of segments showing LGE and the number of non-septal segments were significantly greater in patients with VA, but no difference was found in the number of septal segments involved. Non-septal LGE segments remained significantly related to the occurrence of arrhythmias in a multivariate analysis which included the total number of LGE segments, and so the study pointed to the possibility that location added information beyond fibrosis extent.13 Similarly, in our study we found a correlation between LGE involvement of a specific set of segments – referred to throughout the text as arrhythmogenic segments – and the occurrence of VA.
In HCM, LGE is typically located in the hypertrophied segments of the LV wall.14 Therefore, as expected from the proportion of patients with septal HCM in our sample (45 out of 61), septal segments were those most commonly involved by LGE. However, a group of six segments were more frequently involved in patients with VA than in those without. After multivariate analysis, four of these six segments – basal inferior, basal anterolateral, mid inferoseptal and apical – remained independently associated with the occurrence of VA and so were considered arrhythmogenic segments. LGE extent in these four segments was found to correlate significantly with SCD risk at five years and to independently predict the occurrence of VA. Looking carefully at their location, there appeared to be a relation with the mitral valve apparatus, particularly with the posteromedial papillary muscle insertion and the posterior mitral valve leaflet insertion.
The insertion points of the posterior mitral valve leaflet (which is more loosely anchored and hence moves more freely than that of the anterior leaflet15) and papillary muscle, as well as the apex, are probably the cardiac structures subjected to most hemodynamic stress. Some observational studies suggest that patients with apical aneurysms16 and apical HCM17 represent high-risk subgroups for arrhythmic events. Additionally, a study conducted in patients with mitral valve prolapse showed that LGE at the papillary muscles and inferobasal wall were associated with the occurrence of arrhythmias and correlated with their site of origin.9 It is also noteworthy that in these patients the volume of scarred tissue was small (1.2% of the left ventricle in patients with complex ventricular arrhythmias vs. 0% in those without). The authors hypothesized that mechanical stretch caused by the prolapsing leaflet and elongated chordae could act as a trigger of electric instability.
In addition, studies using positron emission tomography (PET) reporting on normal cardiac fluorine-18 fluorodeoxyglucose uptake patterns found increased activity at the base of the heart, mostly in locations corresponding to the basal inferior and lateral walls in the AHA model.18 Also in PET studies, focal activity in papillary muscles is equally frequent.19 One explanation suggested by the authors of the latter study is an association between increased myocardial wall stress and increased metabolic demand.
Excitation-contraction coupling is a basic principle of cardiac physiology. However, a mechanical stimulus is also capable of inducing electrical activity, as in commotio cordis. This mechanism is known as mechano-electrical feedback20 and appears to be more pronounced in non-uniform cardiac muscle.21 This is a possible explanation for our findings: the occurrence of non-uniform myocardium (demonstrated by LGE at CMR) that is more susceptible to mechanical stimulation in these four arrhythmogenic segments, corresponding to areas of particularly high mechanical stress, confers an increased arrhythmogenic potential in HCM patients.
Study limitationsLGE extent was quantified by visual assessment of the number of segments affected and not by percentage of LV mass. The latter method was not used because LGE quantification is time-consuming in routine daily practice and establishing a quantitative relation between LGE extent and arrhythmic risk was not the aim of our work. Although our results could be explained by a higher percentage of LGE in the arrhythmogenic segments, the fact that the results were the same in asymmetric septal HCM patients, in whom most of the fibrosis is expected to be located in the septum, supports our hypothesis. Additionally, because of the small population and few sudden deaths, replicating the methodology in other small studies22–24 we included non-sustained ventricular tachycardia in the category of ventricular arrhythmias and sudden death. This is a retrospective study that included a small population and so replicating these findings in a larger population, such as in a multicenter study, would be of interest.
ConclusionsIn this retrospective series, the extent of LGE involvement in a set of arrhythmogenic myocardial segments with an apparent relation to cardiac areas of increased mechanical stress was significantly and independently associated with the occurrence of VA in patients with HCM, indicating the possible existence of areas of myocardium with increased arrhythmogenic potential. Larger multicenter prospective studies are needed to confirm this hypothesis, incorporating LGE quantification as a percentage of LV mass and other potential CMR imaging predictors, such as intracavitary flow patterns obtained by four-dimensional flow imaging, perfusion abnormalities, T1 mapping and diffusion tensor-derived parameters.
Conflicts of interestThe authors have no conflicts of interest to declare.