One of the treatments for renal artery stenosis is endovascular intervention, but its effectiveness is controversial. The present study aims to analyze the experience of a working group in the endovascular treatment of selected patients with severe obstructive atherosclerotic lesions of the renal arteries, and to characterize early and late results.
MethodsThis is a retrospective study of symptomatic patients with atherosclerotic renal artery stenosis who underwent endoluminal therapy between May 12, 1999 and March 12, 2015 at two institutions. Statistical analysis was performed using the PASW Statistics program.
ResultsA total of 99 patients were treated, mean age 66 years and 76.8% male. The mean degree of stenosis measured by renal Doppler echocardiography was 83% and 64.6% were ostial lesions. Mean preoperative creatinine level was higher than the postoperative mean: 1.3 vs. 1.2 mg/dl (p=0.014). The number of antihypertensive drugs in the preoperative period was higher than in the postoperative period: 2.0 vs. 1.3 (p=0.001). The mean follow-up was 40 months (0-164). The mean peak systolic velocity over time in the postoperative period was 77 cm/s (40-250). The restenosis rate was 8%, and 30-day mortality was 0%.
ConclusionsThe results demonstrated that the endovascular technique has a beneficial effect on blood pressure and renal function in selected patients, and is a safe technique associated with a high rate of technical success and few complications.
Um dos tratamentos da estenose das artérias renais é a intervenção endovascular sendo a sua eficácia controversa. O presente estudo tem como objetivo analisar a experiência de um grupo de trabalho no tratamento endovascular de doentes selecionados com lesões obstrutivas ateroscleróticas graves das artérias renais, procurando caracterizar os resultados precoces e tardios.
Material e métodosTrata-se de um estudo retrospetivo de doentes com lesões ateroscleróticas das artérias renais, sintomáticos, submetidos a terapêutica endoluminal, de 12 de maio de 1999 a 12 de março de 2015 em duas instituições. A análise estatística foi realizada com o programa PASW Statistics.
ResultadosForam tratados 99 doentes com média de 66 anos e 76,8% homens. O grau de estenose médio foi de 83% medido através do ecodoppler renal sendo 64,6% das lesões ostiais. A média de creatinina no pré-operatório foi superior à média do pós-operatório: 1,3 versus 1,2 (p=0,014). O número de fármacos anti-hipertensores no pré-operatório foi superior ao do pós-operatório: 2,0 versus 1,3, (p=0,001). A média de follow-up foi de 40 meses [0-164]. A velocidade sistólica média ao longo do tempo no pós-operatório foi de 77 [40-250]. A taxa de re-estenose foi de 8% e a mortalidade aos 30 dias foi de 0%.
ConclusõesOs resultados obtidos demonstraram que a técnica endovascular exibe um efeito benéfico ao nível da tensão arterial e função renal num determinado grupo de doentes sendo uma técnica segura associada a uma taxa elevada de sucesso técnico e baixa de complicações.
Renal artery stenosis is caused by atherosclerosis in 90% of cases1 and is a progressive condition.2 The literature suggests that two years after diagnosis, 3%, 18% and 55% of renal function is lost in cases of unilateral stenosis, bilateral stenosis and contralateral occlusion, respectively,3 and that 9% of lesions progress to occlusion at five years.4
Renal artery stenosis may have a significant clinical impact, manifested as arterial hypertension and/or renal dysfunction (RD), and is a contributing factor to increased overall cardiovascular risk.5,6
Renal or surgical endovascular revascularization has been performed frequently since the 1980s in patients with severe stenosis associated with hypertension and/or RD, with good results reported in both individual and institutional series. However, some studies (EMMA, DRASTIC, STAR),7–9 despite their obvious methodological limitations, have cast doubt on its benefit. The lack of advantage of endovascular revascularization compared to medical therapy has also been observed in larger studies (ASTRAL, CORAL)10–13 that have had a major influence on the medical community, but that have also been criticized for serious limitations that may have influenced outcomes and conclusions.14
The present study aims to analyze the experience of a working group in the endovascular treatment of selected patients with severe atherosclerotic obstructive lesions of the renal arteries, and to characterize early and late results regarding the risks of the endovascular technique, the restenosis rate, and its benefits in terms of changes in renal function and blood pressure (BP) control.
MethodsA retrospective descriptive study was carried out of patients undergoing endovascular treatment for atherosclerotic renal artery stenosis between May 1999 and March 2015. All patients were treated at two hospital institutions (Hospital de Santa Maria and Instituto Cardiovascular de Lisboa) by the same working group. A complete survey and analysis of the clinical records of all patients undergoing renal revascularization during this period was performed, excluding those who were treated for non-atherosclerotic lesions as well as those who underwent open surgery.
The decision for revascularization was taken according to the following inclusion criteria:
- –
patients with renal insufficiency (glomerular filtration rate [GFR] <60 ml/min/1.73 m2) or difficult-to-control arterial hypertension, even with optimal medical therapy;
- –
greater than 60% stenosis in at least one renal artery, diagnosed or quantified by Doppler echocardiography (echo Doppler), computed tomography angiography or magnetic resonance angiography, and subsequently confirmed by intraoperative arteriography;
- –
systematic hemodynamic assessment by echo Doppler study, calculating the maximum peak systolic velocity (PSV) and the renal-aortic ratio (RAR) and considering lesions with stenosis >60% (PSV>200 cm/s and RAR<3.5) or >80% (PSV>200 cm/s and RAR>3.5).
GFR was calculated using the CKD-EPI equation:
GFR=141×min (Scr/κ,1)α×max(Scr/κ, 1)-1.209×0.993Age×1.018 [if female]×1.159 [if black].
Patients who showed evidence of significant parenchymal disease, those with marked attenuation or loss of the diastolic component in the renal artery trace or with renal resistance index (RI) >0.7, and those with renal diameter <7 cm were excluded from the treatment. The first-line revascularization method was angioplasty with stenting according to the technique described below.
All lesions were confirmed by arteriography performed intraoperatively. In cases in which preoperative duplex, computed tomographic, or magnetic resonance imaging indicated high-grade lesions and disagreed with the angiographic results, renal artery pressure gradients were also obtained. The angioplasty technique generally used a 6F percutaneous system with a standard renal or double-curve guide catheter being introduced into the aorta through a percutaneous femoral introducer and using the no-touch technique. The renal artery was catheterized with a 0.014-0.018-inch guidewire, and primary opening (without predilation) of a balloon-expandable stent was performed. No embolic protection devices were used. Stent placement, with appropriate opening of the stenotic lesion, was considered a technical success if residual angiographic stenosis was <30%.
Clinical follow-up was performed by the surgical team (in addition to the respective attending physicians), and the patency of the reconstruction was assessed by echo Doppler.
The study assessed the following variables: (1) clinical and lesion characteristics; (2) technical aspects of the endovascular intervention; (3) operative complications; (4) creatinine level before and after the intervention and in follow-up; (5) number of antihypertensive drugs before and after the intervention and in follow-up; (6) PSV in the revascularized artery; and (7) occurrence of restenosis. Restenosis was defined on echo Doppler examination as the reappearance of acceleration at the level of the treated renal artery, with PSV≥200 cm/s.
Statistical analysisThe statistical analysis was performed using PASW Statistics, version 21. The Wilcoxon signed-rank test was used to compare creatinine values and the number of drugs in the pre- and postoperative periods. Restenosis over time was assessed through survival curves. Results were considered statistically significant at p<0.05.
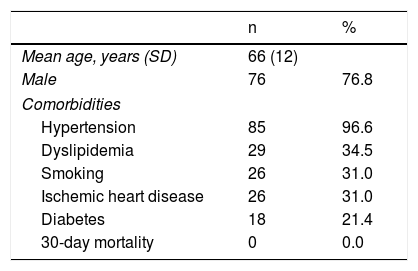
ResultsDuring the study period, 126 renal arteries were treated in 99 patients. In 27 (27.3%) cases the lesions were bilateral and treated at the same time. The mean age of the patients was 66 (40-87) years and 76.8% were male. The most frequent comorbidity was hypertension (96.6%), followed by dyslipidemia (34.5%) (Table 1). Renal artery lesions were located in the ostium in 64.6%, in the proximal third in 29.3%, in the mid third in 4.2% and in the distal two-thirds in 1.2%. The mean degree of stenosis was 83% (70-95%), and all lesions had hemodynamic repercussions on the echo Doppler exam, as mentioned above.
All lesions were confirmed by arteriography performed intraoperatively as currently recommended. In cases in which the preoperative assessment (duplex, computed tomographic, or magnetic resonance imaging) suggested high-grade lesions that were not confirmed by intraoperative angiography no intervention was performed and these cases were not included in the study.
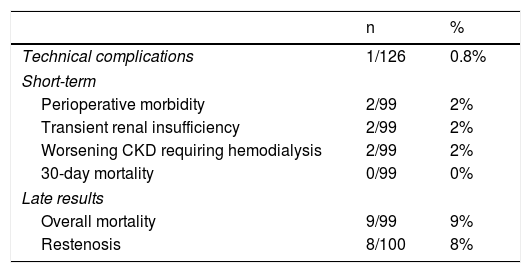
The technical success rate was 99% (125/126). In one bilateral case, catheterization of one renal artery was not possible because of marked angulation. Two perioperative complications occurred: one case of reversible postoperative stroke and one case of iatrogenic rupture of the right renal artery, treated through the placement of a covered stent.15 Thus, the major complication rate was 2% (2/126), the rate of technical complications was 0.8% (1/126) and there was no mortality.
The mean follow-up period was 40 months (0-164).
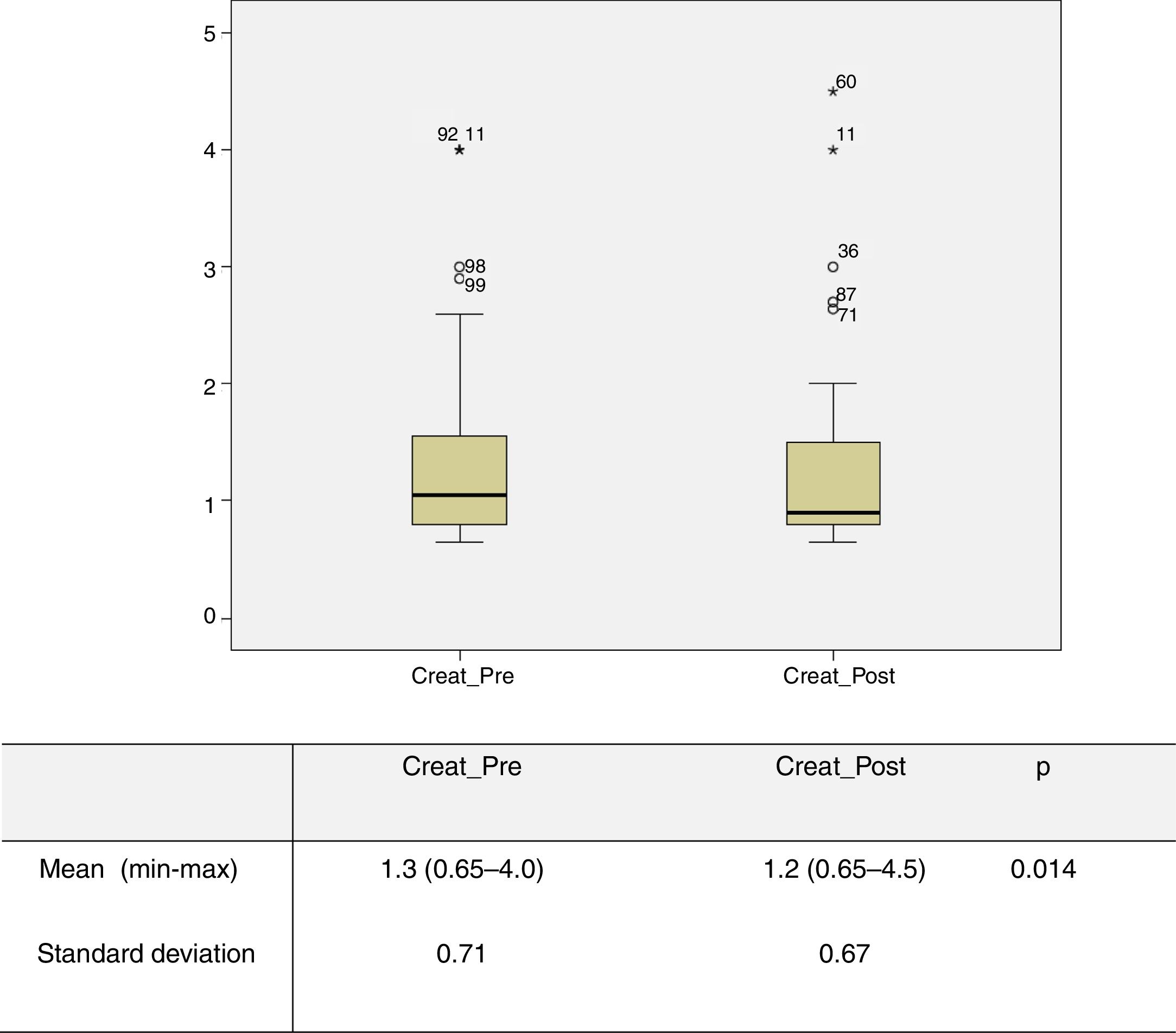
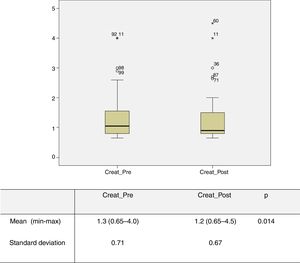
Impact of treatment in the immediate postoperative periodPreoperative and 30-day postoperative serum creatinine levels were obtained for 87 patients. Analysis of the overall sample revealed a mean preoperative creatinine level of 1.3 mg/dl (0.65-4.0), which was higher than the mean postoperative level of 1.2 mg/dl (0.65-4.5) (p=0.014) (Figure 1). The same was observed with GFR, with a preoperative mean of 63.3 ml/min/1.73 m2 (median 61), lower than the postoperative mean of 69.0 ml/min/1.73 m2 (median 79) (p=0.002). Two patients (2%) experienced a transient worsening of creatinine values in the immediate postoperative period, between 0.2 and 1 mg/dl, but these recovered to lower than preoperative levels. However, another two patients (2%) developed rapidly progressive renal failure requiring the initiation of hemodialysis, both 30 days after the procedure, although there was no evidence on echo Doppler of restenosis or renal occlusion. Both initially had very severe chronic kidney disease with preoperative creatinine values of 4 mg/dl.
Graphical representation of preoperative and 30-day postoperative serum creatinine values obtained for 87 patients. Analysis of the overall sample revealed a mean preoperative creatinine level (Creat_Pre) of 1.3 mg/dl (0.65-4.0), which was significantly higher than the mean postoperative level (Creat_Post) of 1.2 mg/dl (0.65-4.5) (p=0.014).
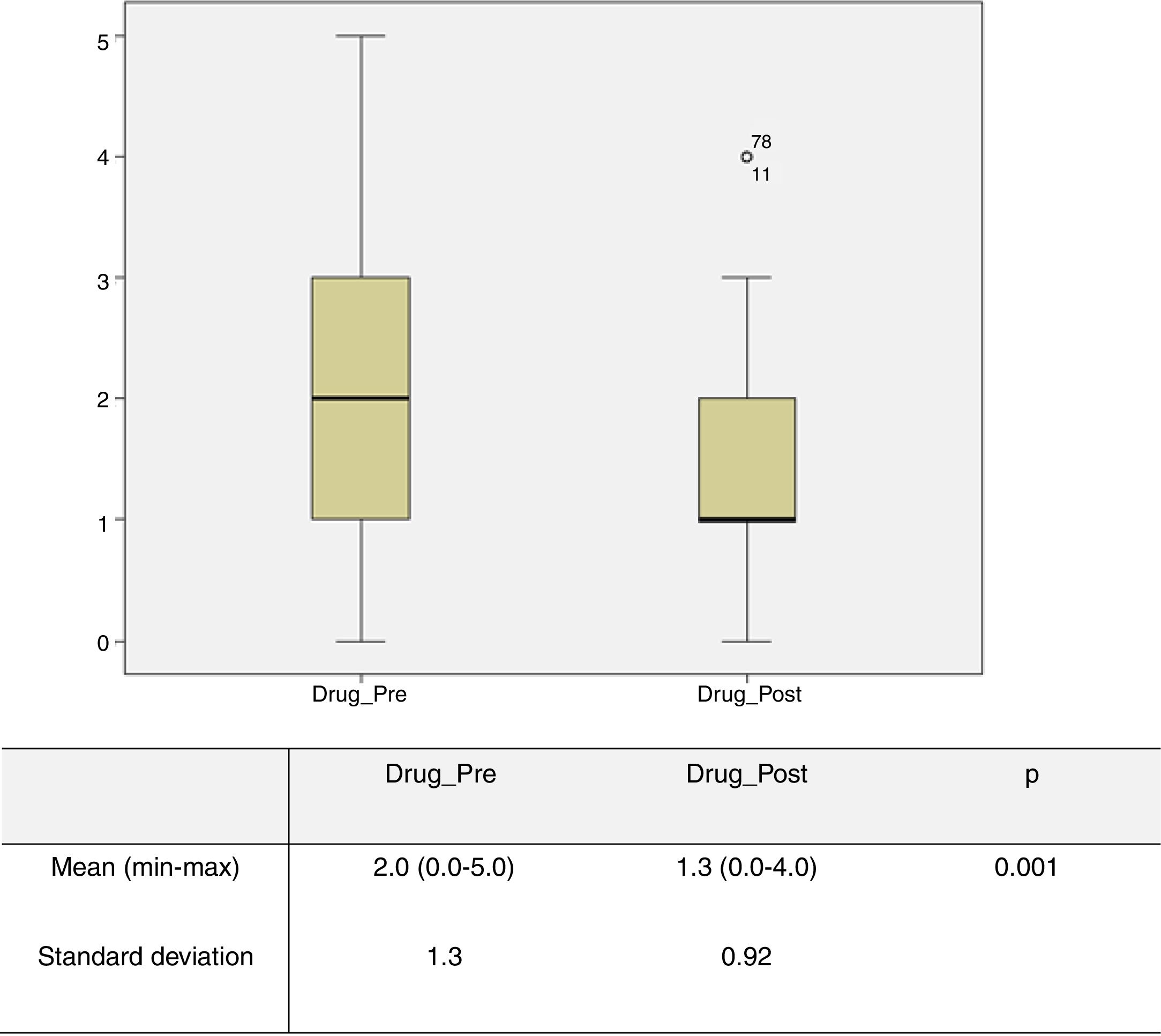
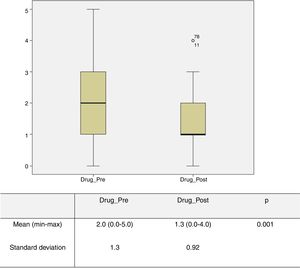
The number of antihypertensive drugs administered preoperatively and 30 days postoperatively was obtained in 72 patients; of these 15 (20%) were under four or five antihypertensives. The mean number of these drugs used in the preoperative period was 2 (0-5), which was significantly higher (p=0.001) than the mean of 1.3 (0-4) drugs used in the immediate postoperative period (Figure 2). Regarding BP control, records were obtained for 87 patients, and in 41.4% of cases BP remained unchanged in the immediate postoperative period, in 46% of cases there was an improvement (defined as achieving BP control with fewer antihypertensive drugs and/or better control with the same number of drugs), and in 12.6% of patients BP control was achieved without medical therapy.
Graphical representation of the number of drugs in the pre- and postoperative period obtained in 72 patients. The mean number of these drugs used in the preoperative period (Drug_Pre) was 2 (0-5), which was significantly higher (p=0.001) than the mean number of 1.3 (0-4) drugs used in the immediate postoperative period (Drug_Post).
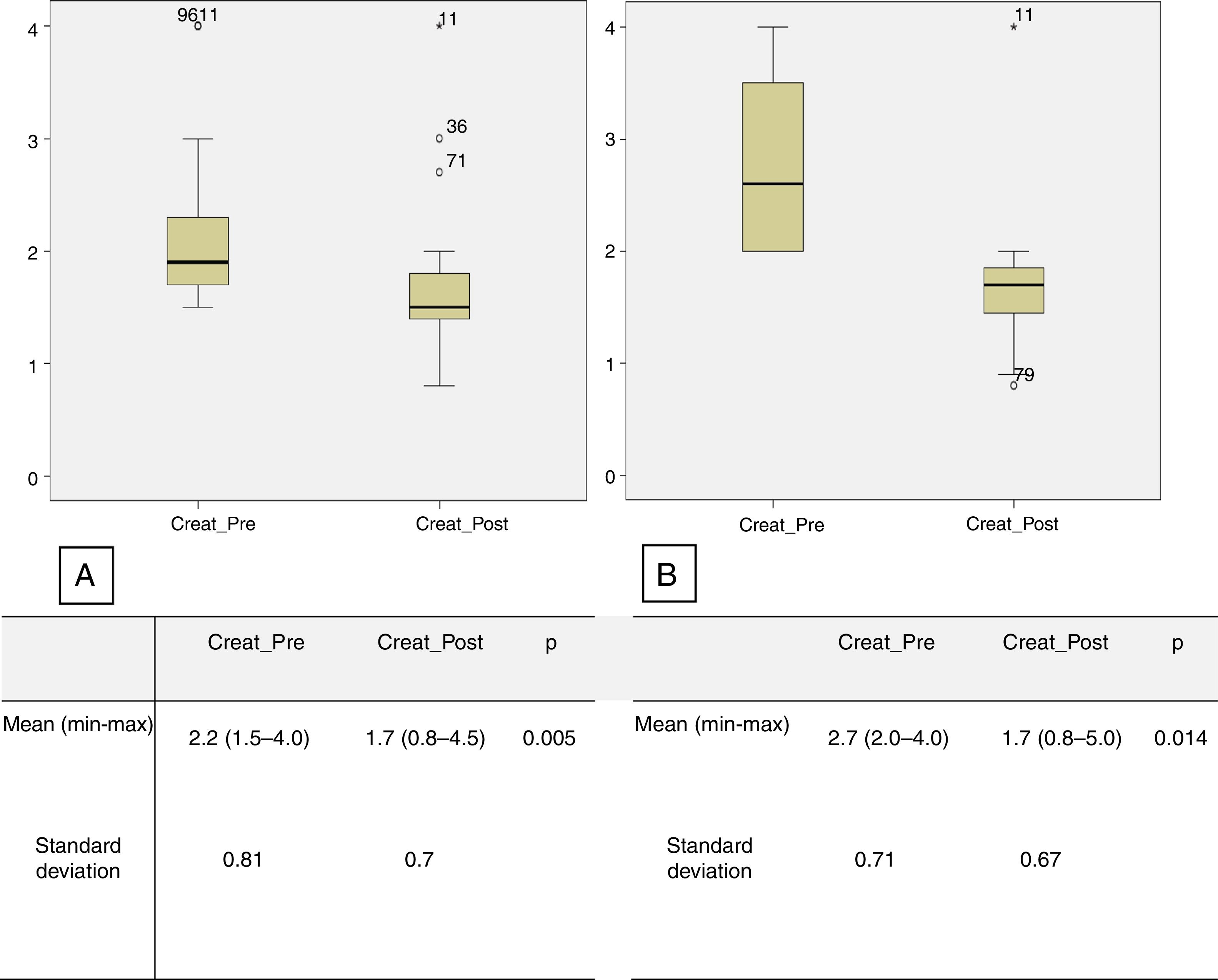
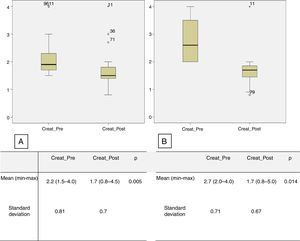
Creatinine levels and the number of antihypertensive drugs were also assessed in the subgroups of patients with RD and preoperative creatinine levels higher than 1.5 mg/dl and 2 mg/dl. There were 23 patients in the first subgroup (creatinine ≥1.5 mg/dl), with mean preoperative creatinine of 2.2 mg/dl (1.5-4.0), decreasing significantly to 1.7 mg/dl (0.8-4.5) in the immediate postoperative period (p=0.005). The mean number of antihypertensive drugs used in the preoperative period was 2.7 (0-5), significantly higher than the number in the immediate postoperative period, which was 1.8 (0-4) (p=0.01) (Figure 3A). In the second subgroup (preoperative creatinine ≥2.0 mg/dl), 11 patients were identified, with mean preoperative creatinine of 2.7 mg/dl (2.0-4.0), decreasing postoperatively to 1.7 mg/dl (0.8-5.0) (p=0.005) (Figure 3B). The mean number of antihypertensive drugs used in the preoperative period was 3.1 (1-5), considerably higher than the mean number used in the immediate postoperative period, which was 2.4 (1-4) (p=0.05).
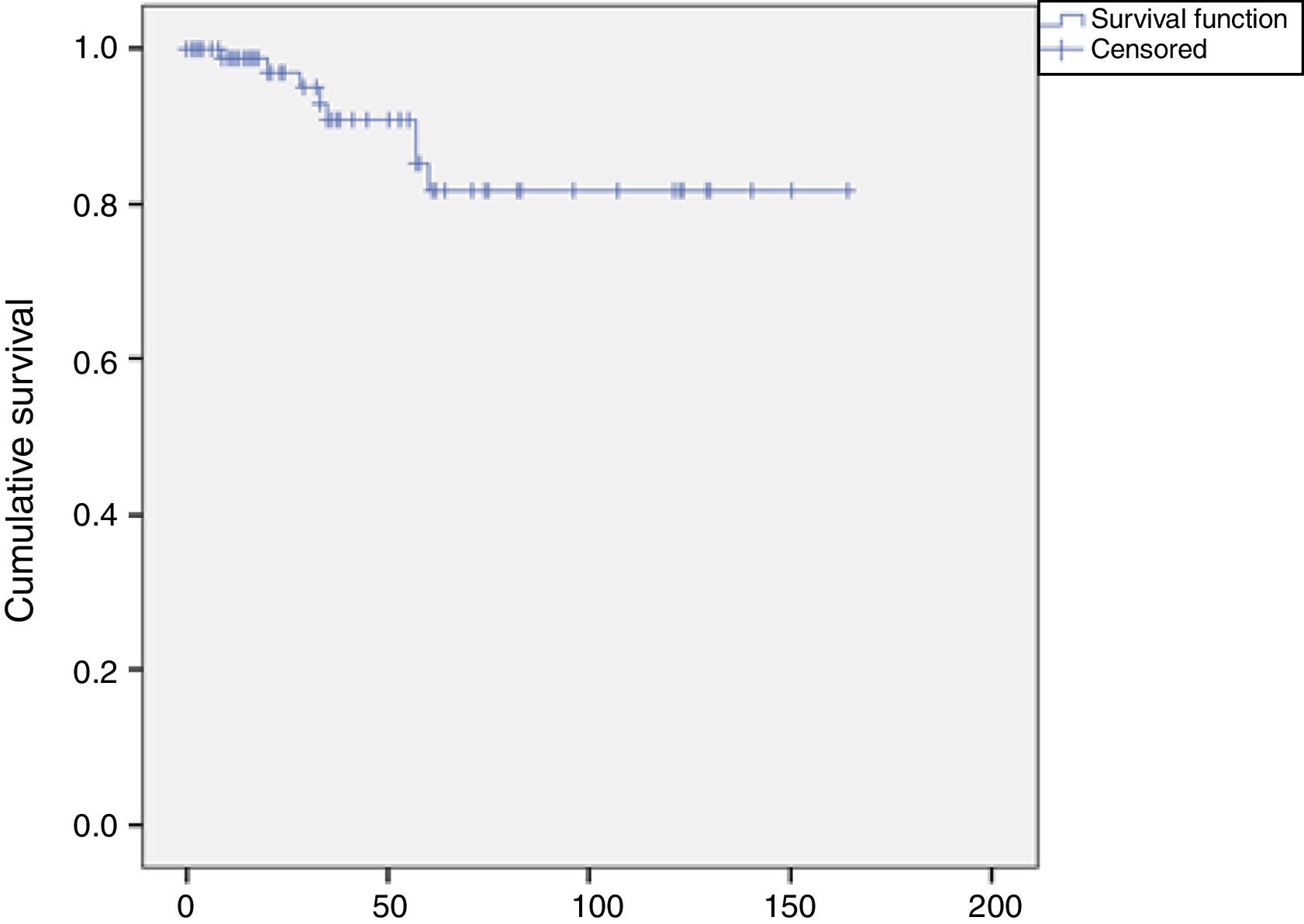
Late results and restenosis rateAs mentioned above, the mean follow-up period was 40 months (0-164). Overall mortality during follow-up was 9% (n=9), and 20 patients were lost to follow-up, which corresponds to 26 treated arteries.
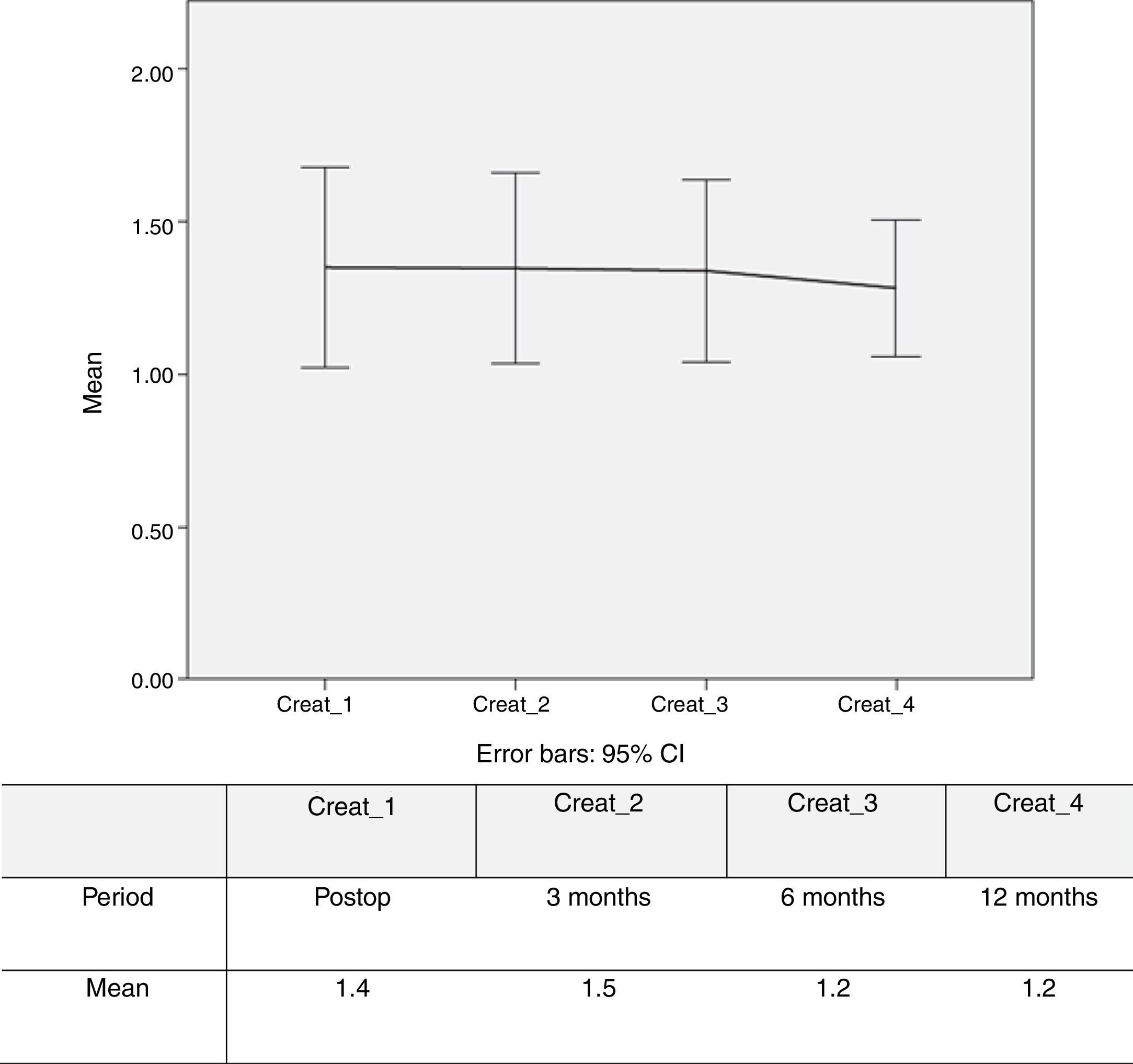
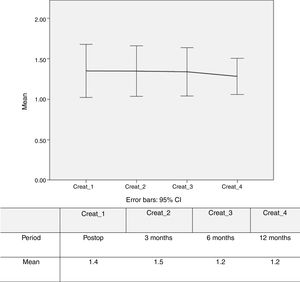
Figure 4 shows stability and slight improvement of mean creatinine levels over the first year of follow-up.
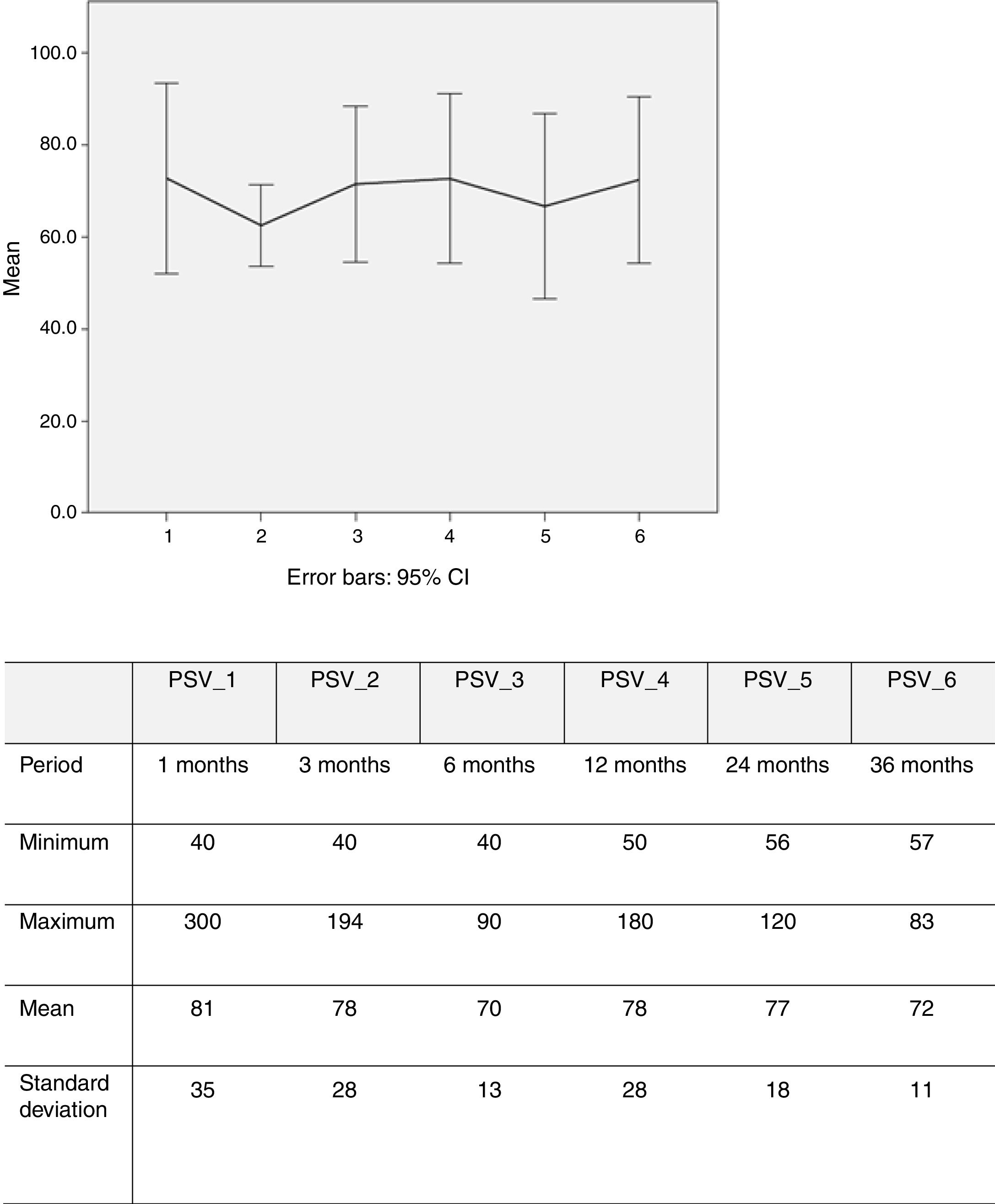
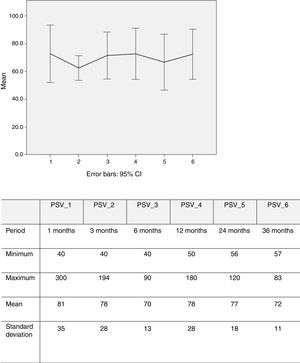
PSV was measured in 100 renal arteries (26 missing). Echo Doppler assessment showed that mean PSV over time was 77 cm/s (40-250) (Figure 5).
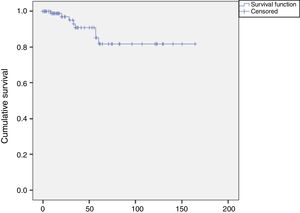
Eight restenosis lesions were identified, a rate of 8% (8/100). The Kaplan-Meier survival curve showed these to have occurred mostly between 25 and 52 months of follow-up (Figure 6). Of these eight patients with significant restenosis, two were reoperated (Table 2).
Morbidity and mortality during follow-up.
| n | % | |
|---|---|---|
| Technical complications | 1/126 | 0.8% |
| Short-term | ||
| Perioperative morbidity | 2/99 | 2% |
| Transient renal insufficiency | 2/99 | 2% |
| Worsening CKD requiring hemodialysis | 2/99 | 2% |
| 30-day mortality | 0/99 | 0% |
| Late results | ||
| Overall mortality | 9/99 | 9% |
| Restenosis | 8/100 | 8% |
CKD: chronic kidney disease.
The present study aimed to review our experience in the endovascular treatment of atherosclerotic renovascular disease and to evaluate its efficacy and safety as well as rates of restenosis and reintervention. The results presented are favorable and suggest an overall benefit.
Progressive decrease in renal artery lumen diameter leads to renal hypoperfusion, which activates the renin-angiotensin-aldosterone system, resulting in renovascular hypertension. However, the pathophysiology differs in unilateral lesions with a functioning contralateral kidney, in which the vasoconstrictive effect of angiotensin II predominates, compared to bilateral or unilateral lesions in a single kidney, in which the main mechanism is volume overload due to the effect of aldosterone.
In addition, decreased renal perfusion causes structural and functional changes in the kidney, known as ischemic nephropathy, which is known to be an important cause of renal failure.
Patients with renovascular hypertension are at higher risk for chronic kidney disease, and several classical studies suggest that about one third of patients with renovascular hypertension develop chronic renal failure within six years, about 17% of hemodialysis patients have uncontrolled renovascular hypertension,16 and 14% are diagnosed with ischemic nephropathy.17,18
Renal revascularization through open surgery was developed in the 1970s and has played an important role in the treatment of patients with occlusive renal artery injuries. However, endovascular therapy applied to the renal territory has replaced open revascularization in most cases because it is less invasive and is applicable to a greater number of patients. At the present time and in atherosclerotic disease, the technical standard consists of the use of balloon-expandable primary stents. The effectiveness of renal angioplasty has been reported in multiple institutional and individual series, with rates of BP improvement of 50-80%,19–21 and improvement or stabilization of renal function in 70-85% of patients.22
However, despite the good results reported with endovascular revascularization in most institutional studies, there remained some controversy regarding the benefits of renal angioplasty in the correction of hypertension and RD after some randomized studies (EMMA, DRASTIC and STAR) produced negative results.7–9 Major level 1 studies (CORA and ASTRAL)11,13 were then conducted, but showed no benefit of endovascular treatment versus medical therapy with regard to progression of renal failure, BP control and overall cardiovascular risk.10–13
Despite the conclusions of these trials and the considerable impact they had on the medical community, methodological limitations were pointed out in both, including the non-consecutive inclusion of all patients (at the discretion of the attending physician) and inclusion of significant numbers of clinically less severe patients (in the ASTRAL and CORAL studies, 41% and 50% of patients had <70% and <80% renal artery stenosis, respectively) and those with moderate injury, as well as high-risk patients and those outside the current standard of endovascular treatment.14 The results of the ASTRAL and CORAL studies do not exclude the possibility that some subgroups of patients may benefit from endovascular intervention (these patients may even have been excluded from ASTRAL and CORAL), as is widely believed by many experts.23
The identification of patients who may benefit from renal intervention has remained a research target in patients with severe bilateral disease, a single kidney, a transplanted kidney, episodes of flash pulmonary edema and those with resistant, malignant or accelerated hypertension when there is a recent decline in renal function and provided that GFR is not less than 25 ml/min. B-type natriuretic peptide >50 pg/ml is also considered predictive of successful revascularization. However, benefit is not expected in cases where the lesions are not hemodynamically significant, so instruments such as the assessment of focal velocity increase on echo Doppler or trans-stenotic pressure gradient >20 mmHg are considered crucial.
The current study has the advantage of including only symptomatic patients (RD and/or difficult-to-control hypertension, even under optimal pharmacological therapy), in which the hemodynamic repercussion of the stenosis was used to determine PSV with echo Doppler, and the treatment had been performed with very low risk. We observed a favorable impact on BP control in 58.6% of patients, which is in line with the literature.20–22 In a 2013 study that combined the results of five multicenter prospective trials, Weinberg et al.24 observed a significant decrease in systolic and diastolic BP after the intervention, with a >10 mmHg reduction in systolic BP in 61% of patients with a higher preoperative baseline systolic BP (>150 mmHg).
Regarding renal function, a significant improvement in serum creatinine levels after the procedure was observed in the present study, especially in the groups that had prior RD. However, it should be taken into account that serum creatinine does not appear to be the ideal marker of renal function, as recognized by some authors,25 and it has been suggested that dynamic measurement of renal function based on the slope of the linear regression line representing serum creatinine versus time may be more accurate.18 Therefore, we also chose to chart changes in creatinine values over time, aiming at a downward sloping line, in agreement with the study by Steichen26 suggesting that the intervention had a positive impact on renal function. Other studies refer to estimated GFR, which has also been criticized for having low sensitivity to detect variations at or above 20%, as well as less rigor in determining changes before and after renal angioplasty.
We observed that in patients with chronic pre-intervention RD (creatinine >1.5 and >2 mg/dl), the improvement in renal function was more significant in the higher creatinine subgroup (>2.0 mg/dl), and this seems to have been maintained over time. This result suggests that the results of endovascular intervention have more impact in patients with greater deterioration of renal function, although it should be noted that patients with atrophic kidney (<7 cm), RI>0.7 or parenchymal disease were excluded from the intervention, as were patients with end-stage renal disease, as long as there was no recent acute decline.
The risk associated with the intervention is another factor to consider when evaluating its benefit. In our study endovascular intervention was performed with a low complication rate (major morbidity of 2% and technical complications of 0.8%) and without mortality, consistent with that reported by a modern center of excellence in which renal angioplasty was associated with greater than 95% technical success, less than 5% major morbidity and less than 1% mortality.27,28
Restenosis is the main limitation of the endovascular technique, and its incidence can reach 10-20%, so patient follow-up is very important.19 In general, renal artery stenting is performed with echo Doppler to identify lesions that will require redilatation, which contributes to assisted primary patency rates of 90-95%.29 In our series, the observed restenosis rate was slightly lower, at 8%, and only two patients required repeat angioplasty. Furthermore, assessment of sequential PSV over the follow-up period is an interesting aspect of the present study and demonstrates relative in-stent hemodynamic stability over time.
The strongest points of the present study are its rigorous patient selection and the marked reduction in risk obtained with endovascular intervention. As limitations, we should point out that the study was retrospective in nature and that renal function was only assessed through creatinine levels. Furthermore, assessment of changes in BP was based on the number of antihypertensive drugs, and it was impossible to know the types and doses of these drugs for all patients. Finally, BP measurements were not always taken with the same periodicity.
In conclusion, in the present study, the technical success of renal artery stenting was high, adverse events were infrequent, and clinical success and durability, at least in the short term, were positive. Given the quality of the available evidence, renal revascularization is clearly beneficial in selected patients. Thus, according to our experience, renal revascularization should be indicated in the following cases:
- •
stenotic renal artery lesions with demonstrated hemodynamic repercussion;
- •
severe bilateral or single kidney injury;
- •
severe single or bilateral lesions in patients with malignant, accelerated, resistant or difficult-to-control hypertension, ipsilateral renal atrophy (but with renal diameter >6 cm), or renal impairment in patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers;
- •
severe bilateral or single kidney injury in patients with progressive renal failure or with flash pulmonary edema.30
Renal stenting should be the first-choice technique since it is associated with low risk. Future research should be encouraged to identify patients who will best respond to this therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.