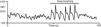Knowledge of the physiology underlying the autonomic nervous system is pivotal for understanding autonomic dysfunction in clinical practice. Autonomic dysfunction may result from primary modifications of the autonomic nervous system or be secondary to a wide range of diseases that cause severe morbidity and mortality. Together with a detailed history and physical examination, laboratory assessment of autonomic function is essential for the analysis of various clinical conditions and the establishment of effective, personalized and precise therapeutic schemes. This review summarizes the main aspects of autonomic medicine that constitute the background of cardiovascular autonomic dysfunction.
O conhecimento subjacente à fisiologia do sistema nervoso autónomo é fundamental para se entender a disfunção autonómica na prática clinica. A disfunção autonómica pode resultar primariamente de modificações do sistema ou, secundariamente, a uma série de patologias conducentes a morbilidade ou mortalidade. Juntamente com a colheita detalhada da história clínica e do exame objetivo, a avaliação autonómica laboratorial torna-se essencial na análise de algumas condições clínicas e no estabelecimento de esquemas terapêuticos mais eficazes, refinados e personalizados. Assim, nesta revisão sumarizam-se os aspetos mais relevantes da fisiologia autonómica subjacente a disfunção autonómica cardiovascular.
Attempts to bridge the gap between basic and clinical science, known as the translational approach to medical knowledge, contribute to better clinical practice, enabling a comprehensive interpretation of pathophysiological mechanisms, more accurate diagnosis and establishing more effective treatment. Advances in autonomic research in recent years and the development of implantable devices that affect autonomic tone mean there is a growing need to understand the scientific basis of autonomic medicine, in order to improve management of autonomic dysfunction in cardiology.
This review aims to provide a basis for understanding autonomic failure in cardiovascular disease. The first section deals with the basic aspects of autonomic function, while the second covers the most important reflexes. The third section describes the most common methods of autonomic evaluation.
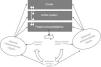
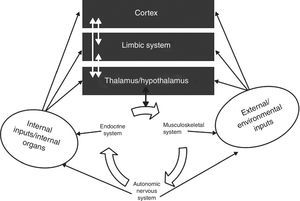
The autonomic nervous systemAlmost all bodily functions are dependent on the autonomic nervous system (ANS), which exerts precise control over visceral functions (Figure 1). However, the mechanisms through which the ANS exerts this control are not well understood.
The interactions between the autonomic nervous system, the brain, the body and the environment. Adapted from Jänig.16
Although the ANS is able to hide its own dysfunction, disautonomy, also known as autonomic failure, can occur due to functional failure, a physical defect in the nervous network, and as a result of the aging process. In these conditions, the system becomes over-activated, the resulting allostatic overload being believed to contribute to various diseases, including hypertension, atrial fibrillation and other cardiac arrhythmias, ischemic heart disease, obesity, diabetes, atherosclerosis, sleep apnea, metabolic syndrome and congestive heart failure.1–14
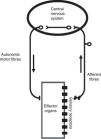
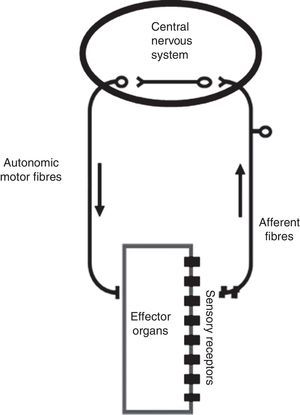
The ANS is one of the two major divisions of the peripheral nervous system (the other being the somatic nervous system). The ANS functions mainly through negative feedback mechanisms and via reflex arcs, using specific neuronal pathways in the periphery and a specific central organization to perform precise and flexible actions. In the present text, we will use Langley's neuroanatomical terminology and the terms sympathetic, parasympathetic and enteric will only refer to the motor portion of the autonomic reflex arc (Figure 2). This arc also includes integrative centers located in the central nervous system (CNS) (the central autonomic network) to which sensory information is conveyed from peripheral sensors located in specific reflexogenic areas.6,12,14,15
The autonomic reflex arc. The morphological relations between its different components are shown. The autonomic motor fibers include sympathetic, parasympathetic and enteric fibers. Adapted from Rocha.106
Afferent pathways are the interface between the visceral organs and the CNS. Most afferent fibers are unmyelinated, but myelinated fibers can also conduct autonomic sensory information.15 There are two types of visceral afferents, primary afferent fibers and enteric afferent fibers. The latter respond to chemical and mechanical events and their cell bodies are located in the gastrointestinal tract walls.15,16 Primary afferent inputs are carried orthodromically to the spinal cord, brain stem or prevertebral sympathetic ganglia. The degree to which these afferent neurons are physiologically specific is determined by the responses evoked by chemical or mechanical stimulation.15–17 Most of these afferents transmit information from the viscera to the CNS,4,18–20 but some also make contact with sympathetic preganglionic neurons in the prevertebral ganglia.21 These anatomical relations indicate that in addition to their central actions, visceral primary afferents (and also enteric afferent fibers) may also play a role in peripheral regulatory reflexes, mainly those that are active in pathological conditions through positive feedback mechanisms.21
Afferent neurons are involved in two main functions: regulation of visceral actions, including organ-protective reflexes; and the transport of pain information, including pain from deep somatic tissues and regulation of hyperalgesia, deep pain and inflammation.15,16,22 This dual function makes them fundamentally different from somatic afferents, in which sensory and regulatory properties cannot be separated; afferent impulses from skin or muscle trigger reflexes and behavioral regulation simultaneously with sensory experience, which is not the case with visceral afferents, as some stimuli from the latter never reach the level of consciousness, such as changes in blood pressure (BP) or gut distension.15–17,23 The majority of the viscera show dual afferent innervation, with most afferent fibers traveling in mixed parasympathetic nerves, such as the vagus and pelvic nerves.24 The physiological significance of this dual innervation – afferent fibers being carried in sympathetic and parasympathetic nerves – is not fully understood, but data suggest that reflex and regulatory functions evoked by visceral stimulation are mainly triggered by activity in afferent fibers in the vagus and pelvic nerves, while visceral sensation, and in particular visceral pain, together with some visceral reflexes originating in the mesenterium, are mediated by afferent fibers in sympathetic nerves.15,16
Efferent pathwaysEfferent pathways of the peripheral ANS have three major subdivisions: sympathetic, parasympathetic, and enteric. These systems are the building blocks of the motor part of the autonomic reflex arc and establish the final autonomic pathway,25 as each of them consists of a series of pre- and postganglionic neurons that are synaptically connected in autonomic ganglia, which function as the connection between the brain centers and the target organ.
Each autonomic nerve pathway extending from the CNS to an innervated organ is a two-neuron chain (except to the adrenal medulla that in effect functions as a sympathetic ganglion). The cell body of the first neuron, located in the CNS, synapses with a second-order neuron, the cell body of which lies within an autonomic ganglion.26 It is generally accepted that, except for the enteric nervous system, the organization of parasympathetic nervous pathways is simpler than that of the sympathetic nervous system. However, while this may be true for some pathways and target organs such as the pupillae and ciliary muscles, it seems unlikely to be the case for other target organs like the heart or the urinary bladder.13,27–29
Sympathetic efferent pathwaysSympathetic preganglionic neurons are a heterogeneous population. Morphologically, they vary in somal shape, size and dendritic arborization, giving rise to either non-myelinated or myelinated axons, which are not selective in relation to a single target. In the spinal cord, sympathetic preganglionic neurons are located in four nuclei: the lateral funicular, intermediolateral, intercalated, and central autonomic nuclei, of which the most important for cardiovascular regulation is the intermediolateral nucleus.
Independently of how preganglionic sympathetic neurons are positioned within the different spinal nuclei, they are segmentally organized, this arrangement providing the anatomical substrate for a more general rostrocaudal functional topography.15,30
Sympathetic preganglionic neurons exhibit a low level of tonic activity. This may reflect the influence of both intrinsic membrane properties and the integration of excitatory and inhibitory postsynaptic potentials. Their activity is regulated by segmental inputs from visceral and somatic afferents and supra-spinal pathways.31 According to their biophysical properties and functional properties, sympathetic preganglionic neurons can be divided into phasic (rapidly adapting), tonic (slowly adapting), and those with a long after-depolarization.
Parasympathetic efferent pathwaysCompared to the quantity of research on sympathetic reflex responses, there have been relatively few studies on the parasympathetic system. There are various reasons for this but they are all due to the fact that most parasympathetic ganglia are located close to or within the target organ walls, and therefore the postganglionic parasympathetic neuron is very short. These less easily morphologically-defined neuronal structures, together with less pronounced target organ parasympathetic innervation, hamper neural recording and peripheral modulation of parasympathetic circuits.32,33
In neuroanatomical terms, brain stem parasympathetic preganglionic nuclei include the Edinger-Westphal nucleus, the superior and inferior salivatory nuclei, the dorsal motor vagal nucleus and the nucleus ambiguus. Neurons of the ventrolateral nucleus ambiguus provide the main parasympathetic innervation of the cardiac ganglia, which innervate the heart, esophagus, and respiratory airways.34
The heart is innervated by at least two parasympathetic pathways. One, which acts directly on the sinus node and other pacemaker cells, is involved in heartbeat regulation and atrial inotropism. These myelinated neurons emerge from the nucleus ambiguus and are activated by baroreceptor stimulation. They can show spontaneous rhythmic activity, the absence of activity coinciding with inspiration and activation being simultaneous with expiration. This coupling to central respiratory activity is the basis of respiratory sinus arrhythmia. The second pathway is formed mainly of unmyelinated neurons that originate in the dorsal motor nucleus of the vagus. Some of these neurons can also show spontaneous activity that is not modulated by the central respiratory drive or by baroreceptor activity; in the heart, their main function appears to be to induce coronary vasodilation when activated.35–38
Dual autonomic innervationThe two divisions of the ANS rarely operate independently, and autonomic responses generally represent the regulated interplay of both divisions (Table 1). The heart, glands and smooth muscles are innervated by both sympathetic and parasympathetic fibers (dual innervation). Moreover, they are usually activated reciprocally, i.e. when the activity of one division increases, the activity of the other decreases. Dual innervation by nerve fibers that cause opposite responses provides a fine degree of control over the effector organ. The sympathetic system promotes responses that prepare the body for strenuous physical activity in emergency or stressful situations, the sympathetic response being characterized by tachycardia, hypertension and increased blood flow to the skeletal muscles, heart, and brain, release of glucose by the liver and dilatation of the pupils.26,30 The parasympathetic system dominates in quiet, relaxed situations; under nonthreatening circumstances, the body can perform its own general housekeeping activities.26
Some effects of autonomic nervous system activity. Adapted from Vander et al.30
| Effector organ | Receptor type | Sympathetic effect | Parasympathetic effect |
|---|---|---|---|
| Eyes | |||
| Iris muscle | Alpha | Contracts radial muscle (widens pupil) | Contracts sphincter muscle (makes pupil smaller) |
| Ciliary muscle | Beta | Relaxes (flattens lens for far vision) | Contracts (allows lens to become more convex for near vision) |
| Heart | |||
| Sinoatrial node | Beta | Increases heart rate | Decreases heart rate |
| Atria | Beta | Increases contractility | Decreases contractility |
| Atrioventricular node | Beta | Increases conduction velocity | Decreases conduction velocity |
| Ventricles | Beta | Increases contractility | Decreases contractility slightly |
| Arterioles | |||
| Coronary | Alpha | Constricts | – |
| Beta | Dilates | ||
| Skin | Alpha | Constricts | – |
| Skeletal muscle | Alpha | Constricts | – |
| Beta | Dilates | ||
| Abdominal viscera | Alpha | Constricts | |
| Beta | Dilates | – | |
| Salivary glands | Alpha | Constricts | Dilates |
| Veins | Alpha | Constricts | – |
| Beta | Dilates | ||
| Lungs | |||
| Bronchial muscle | Beta | Relaxes | Contracts |
| Bronchial glands | Alpha | Inhibits secretion | Stimulates secretion |
| Beta | Stimulates secretion | ||
| Salivary glands | Alpha | Stimulates watery secretion | Stimulates watery secretion |
| Beta | Stimulates enzyme secretion | ||
| Stomach | |||
| Motility, tone | Alpha and beta | Decreases | Increases |
| Sphincters | Alpha | Contracts | Relaxes |
| Secretion | Inhibits (?) | Stimulates | |
| Intestine | |||
| Motility | Alpha and beta | Decreases | Increases |
| Sphincters | Alpha | Contracts (usually) | Relaxes (usually) |
| Secretion | Alpha | Inhibits | Stimulates |
| Gallbladder | Beta | Relaxes | Contracts |
| Liver | Alpha and beta | Glycogenolysis and gluconeogenesis | – |
| Pancreas | |||
| Exocrine glands | Alpha | Inhibits secretion | Stimulates secretion |
| Endocrine glands | Alpha | Inhibits secretion | – |
| Beta | Stimulates secretion | ||
| Fat cells | Alpha and beta | Increases fat breakdown | – |
| Kidneys | Beta | Increases rennin secretion | – |
| Urinary bladder | |||
| Bladder wall | Beta | Relaxes | Contracts |
| Sphincter | Alpha | Contracts | Relaxes |
| Uterus | Alpha | Contracts in pregnancy | Variable |
| Beta | Relaxes | ||
| Reproductive tract (male) | Alpha | Ejaculation | Erection |
| Skin | |||
| Muscles causing hair erection | Alpha | Contracts | – |
| Sweat glands | Alpha | Localized secretion | Generalized secretion |
| Lachrymal glands | Alpha | Secretion | Secretion |
There are several exceptions to the general rule of dual reciprocal innervation by the two branches of the ANS. Innervated blood vessels (most arterioles and veins) receive only sympathetic nerve fibers. Regulation is accomplished by increasing or decreasing the firing rate above or below the tonic level in these sympathetic fibers. The only blood vessels to receive both sympathetic and parasympathetic fibers are those supplying the penis and clitoris. Most sweat glands are innervated only by sympathetic nerves. Salivary glands are innervated by both autonomic divisions, but sympathetic and parasympathetic activity are not antagonistic; both stimulate salivary secretion, but saliva volume and composition differ depending on which autonomic branch is dominant.26
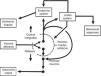
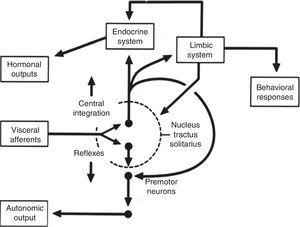
Central autonomic networkCentral autonomic pathways are organized at two levels, some for reflex adjustments of the end organ, while others are organized in a more complex fashion by connecting to higher neural centers, forming a central autonomic circuit capable of producing wide-ranging autonomic, endocrine, and behavioral responses (Figure 3).15,31
Diagram of the two main types of visceral information processing by the central autonomic network. Information originating in the periphery is processed and produces either a reflex response or integrated autonomic, hormonal and behavioral output, the classic example of which is thermoregulation in the hypothalamus. Adapted from Loewy and Spyer.15
Central control of autonomic function involves several interconnected areas distributed throughout the neuraxis.39 This central autonomic network has a critical role in moment-to-moment control of visceral function, homeostasis, and adaptation to internal or external challenges.14,15,31,39
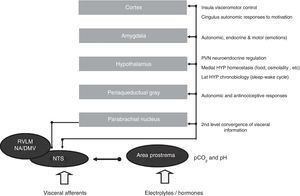
The network functions on four closely interconnected hierarchical levels: spinal, bulbopontine, pontomesencephalic and forebrain (Figure 4). Of these, the bulbopontine level is involved in reflex control of circulation and respiration, and is the location of the nucleus tractus solitarii (NTS), the first relay station for reception of peripheral visceral information, and the rostroventrolateral medulla (RVLM), which contains bulbospinal neurons that are fundamental for the control of vasomotor, cardiac and respiratory function and for the coordination of various cardiovascular reflexes. These RVLM neurons also control hypothalamic function and neurons of the ventral respiratory group involved in respiratory rhythmogenesis.14,15,31,39 Located more rostrally, the parabrachial nucleus is a major relay center for the convergence of various types of sensory information (visceral, nociceptive and thermoreceptive), and contains separate subnuclei linked to gastrointestinal, cardiovascular and respiratory regulation together with clusters of neurons involved in osmo- and thermoregulation.14,15,31,39 The midbrain periaqueductal gray integrates autonomic, somatic and antinociceptive responses to stressful stimuli. Morphologically, it is divided into columns, which control cardiorespiratory and urinary function as well as pain, thermoregulation and reproductive function.14,15,31,39
Central autonomic control areas and levels of interaction of autonomic control. DMV: dorsal motor nucleus of the vagus; HYP: hypothalamus; Lat HYP: lateral hypothalamus; NA: nucleus ambiguous; NTS: nucleus tractus solitarii; PVN: paraventricular nucleus of hypothalamus; RVLM: rostral ventrolateral medulla.
The forebrain level includes the hypothalamus and components of the anterior limbic circuit, including the insular cortex, anterior cingulate cortex and amygdala. Playing a central role in neuroendocrine integration critical for homeostasis and integrative adaptive responses, the hypothalamus and periaqueductal gray are also involved in the defense reaction, an acute but active adaptation to stressful stimuli leading to sympathetic activation and tachycardia, hypertension, positive inotropism, increased stroke volume and cardiac output, redistribution of blood flow, tachypnea, baroreflex inhibition and facilitation of the chemoreceptor reflex.40 In this reaction, the paraventricular hypothalamic nucleus coordinates neuroendocrine integration, including sympathoexcitation, secretion of vasopressin (VP) and activation of the adrenomedullary and adrenocortical systems.
Autonomic cardiovascular reflexesCardiovascular autonomic reflexes include short-term, moment-to-moment, mechanisms that regulate heart rate (HR) and BP. They result from activation of peripheral receptors whose afferents link to the CNS via the glossopharyngeal and vagus nerves.6 CNS processing of afferent information is followed by regulation of autonomic efferent pathways and adjustment of cardiovascular parameters.41 Central BP control involves both sympathetic and parasympathetic nervous systems. The sympathetic system increases stroke volume, HR and total peripheral resistance, increasing BP, while parasympathetic activation decreases HR, cardiac inotropism and stroke volume, reducing BP.42 Several receptor types are involved in the modulation of sympathetic and parasympathetic activity, including arterial baroreceptors, cardiopulmonary receptors and arterial chemoreceptors.6,43–48
Baroreceptor reflexThe baroreceptor reflex is the major mechanism for adjusting BP. It is initiated by stimulation of arterial baroreceptors, which are nerve endings found in vascular and cardiac reflexogenic areas that are sensitive to stretching during the cardiac cycle. In blood vessels, the most important baroreceptors are located in the carotid sinus, aortic arch and mesenteric circulation.49 The aortic arch receptors and medullar cardiovascular centers communicate through the vagus nerve, while it is Hering's nerve, a branch of the glossopharyngeal nerve, that conveys information from the carotid sinus receptors.50 Arterial baroreceptors play a key role in short-term adjustments of BP, maintaining it within a normal range by acting on cardiac output, peripheral resistance and inotropism.51,52
Baroreceptors respond to the distension and deformation that local BP changes elicited by phases of the cardiac cycle induce in the vessel and that change the frequency of nerve impulses that are carried to the NTS.53,54 Changes in baroreceptor activity also affect breathing. As an example, in vivo studies on anesthetized vagotomized dogs showed that the carotid body chemoreceptor reflex response was eliminated by surgically excluding the carotid bodies from the carotid sinus baroreceptor area.55
Baroreceptors are also involved in secretion of VP,56 particularly in response to hypotension, possibly due to neuronal projections from the NTS to the hypothalamus.57 When the baroreceptor reflex is activated by a reduction in BP, an increase in VP secretion is observed,58,59 and intact arterial baroreceptors are essential to maintain BP and VP secretion.60,61 However, in this situation, their action is reinforced by facilitation of the chemoreceptor reflex in the NTS, due to the nature of the stimulus.40
Several studies have examined the role of the baroreceptor reflex in the long-term regulation of sympathetic activity, as central resetting of the baroreceptor-sympathetic reflex may be an important component of the mechanism causing sustained changes in renal sympathetic activity. However, little is known about the mechanisms that could cause such resetting.62
Chemoreceptor reflexArterial chemoreceptors are highly specialized cells that can detect changes in the partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2) and pH in the blood. Peripheral chemoreceptors are more sensitive to changes in pO2 than in pCO2 or pH, while central chemoreceptors respond primarily to changes in pCO2 and pH.42
Peripheral chemoreceptors are mainly located in the aortic and carotid bodies, but may also be found in the mesenteric circulation. The carotid bodies are located bilaterally at the bifurcation of the common carotid artery, while aortic bodies are located between the pulmonary artery and the aortic arch.50 Carotid bodies are more sensitive to hypoxia and hypercapnia, and detect changes in blood gas tension, while aortic baroreceptors are more sensitive to anemia, carboxyhemoglobinemia and systemic hypotension.63 Thus, carotid bodies monitor the ventilation/perfusion ratio and aortic bodies are responsible for reflex control of systemic vascular resistance. Central chemoreception was initially localized to areas on the ventral medullary surface in the area prostrema, but there is substantial evidence that many sites participate in central chemoreception, some located at a distance from the ventral medulla.64
Changes in the partial pressure of gases or pH detected by peripheral chemoreceptors are sent to the NTS through the vagus or the glossopharyngeal nerve.65 Stimulation of chemoreceptors increases the activity of NTS cells. These cells simultaneously excite neurons in the vagal nuclei and RVLM, leading to an increase in sympathetic and parasympathetic tone, which results in ventilatory adjustments – increased air flow volume, respiratory rate and breathing volume – that play an important role in the reflex control of ventilation.66 In addition to ventilatory responses, chemostimulation also induces changes in the cardiovascular system such as tachycardia and vasoconstriction that maintain the chemical composition of the blood and tissue perfusion at optimal levels.
It has been suggested that peripheral chemoreceptors have a tonic excitatory influence on cardiovascular control due to sympathetic activation, thus contributing to maintenance of BP levels.67
Cardiac reflexesThere are also volume receptors located in the right atrium and venae cavae that respond to blood volume decreases by reducing their firing rates. The afferents of these receptors join the vagus nerve and terminate in the NTS, synapsing with neurons projecting to the PVN.68 This pathway is activated by changes in blood volume of as little as 8-10%.69,70 Overall, activation of atrial receptors induces inhibition of sympathetic vasomotor tone and an increase in VP secretion, affecting renal function.
Other cardiac reflexes that regulate BP are the Bainbridge and Bezold-Jarisch reflexes. In the Bainbridge reflex, BP is indirectly regulated through HR changes. When right atrial volume increases, low-pressure stretch receptors initiate a reflex that increases HR through sympathetic nerve activation.63 The Bainbridge reflex is not always active, its effect depending on HR, being stronger at lower than at higher HR values. In this way, the Bainbridge reflex acts in opposition to the baroreceptor reflex, which increases HR when stretching is decreased in hypotension or hypovolemia.71 The Bezold-Jarisch reflex is a cardiac reflex that is sensitive to chemicals, evoking a strong cardiovascular depressor response leading to bradycardia and hypotension as a direct consequence of chemical stimulation of receptors in the ventricles or coronary circulation. The fall in BP is due to both bradycardia and vasodilation caused by inhibition of sympathetic vasomotor activity, and is also modulated by renin release and VP secretion.72 Conversely, decreases in the activity of these inhibitory sensory receptors increase sympathetic activity, vascular resistance, plasma renin activity and VP secretion.72
Evaluation of the autonomic nervous systemThere are various standard provocative autonomic maneuvers designed to test the ANS with stimuli ranging from suprathreshold to maximum intensity, in order to observe evoked responses in target organs in terms of presence or absence, duration and magnitude (Table 2 and Figure 5). These maneuvers should be performed in a dedicated autonomic laboratory, which should have controlled temperature and humidity (20-23°C and 25-35%, respectively) and an area of ∼20 m2. Depending on the type of assessment, each patient may undergo two or more different tests. All tests should be performed under medical supervision by experienced technicians, whose training is critical to the success of any autonomic test battery.11,14 These technicians must be familiar with sudomotor function, electrocardiography, beat-to-beat BP, blood flow recordings and computers, and must be able to identify and solve technical problems and recognize the main electrocardiographic abnormalities, as well as be knowledgeable in electrical safety and be trained in cardiopulmonary resuscitation.11,14
Summary of the provocative maneuvers for autonomic evaluation most commonly used in clinical practice.
| Cardiovascular | Mixed maneuvers: head-up tilt (60°/70°); active standing; Valsalva maneuver Addressing mainly sympathetic branch: handgrip (isometric contraction), cold pressor test, mental arithmetic test Addressing mainly parasympathetic branch: deep breathing Liquid meal challenge Carotid sinus massage |
| Biochemical | Plasma noradrenaline, urinary catecholamines; plasma renin activity and aldosterone |
| Pharmacological | Noradrenaline–α-adrenoceptors, isoprenaline–β-adrenoceptors, atropine |
| Sudomotor | Thermoregulatory sweat test for central regulation Quantitative sudomotor axon reflex test for quantitative local sweat evaluation Silicone imprint with atropine iontophoresis for semi-quantitative local sweat evaluation |
| Eye | Schirmer's test Pupillary function tests Intraocular pressure tests |
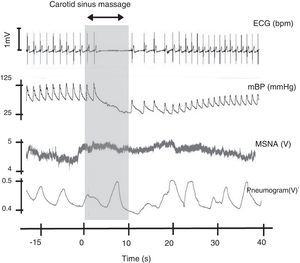
Carotid sinus massage. This figure shows the relation between cardiovascular variables, ventilation and muscle sympathetic nerve activity on sinus massage in a healthy subject. ECG: electrocardiogram; mBP: mean blood pressure; MSNA: muscle sympathetic nerve activity. Rocha and Laranjo, unpublished data.
Patients undergoing autonomic evaluation should not consume food or tobacco at least four hours, and alcohol at least 12 hours, before the test, which should be performed during the morning, and should wear light clothing. Medications, particularly those that directly affect the ANS, should be discontinued according to the drugs’ half-life and the patient's condition. In view of the considerable intra- and inter-individual variability in data, normal values are set by each laboratory and should be grouped by gender, age and decade of life. There are different ways of categorizing autonomic tests that take into account the target system, the type of variables recorded and the degree of invasion. Usually, due to the nature of the recording devices, most maneuvers target the cardiovascular system and are non-invasive in nature.
Autonomic maneuvers and the Ewing test batteryThe evaluation and data analysis protocols should be appropriate to the study. A standard and the most common evaluation protocol is the Ewing test battery,14 which assesses HR response to deep metronomic breathing, BP response to sustained handgrip and BP and HR response to the Valsalva maneuver and active standing.11,14,73,74 Other non-invasive maneuvers, including the cold pressor test, mental stress test and tilt table, are also used in autonomic evaluation.
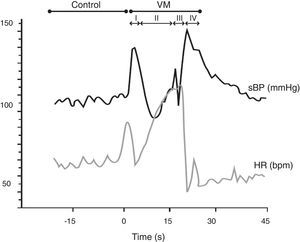
In the Valsalva maneuver, which assesses the sympathetic and parasympathetic reaction to baroreflex activation, the subject maintains an expiratory pressure of 40 mmHg/15 s with an open glottis. The test response is divided into four phases, two of which are reflex in nature (II and IV) and two mechanical (I and III). The results depend on the subject's position, age and gender, as well as the duration and intensity of expiratory pressure. In patients with autonomic dysfunction, there is typically a loss of both BP overshoot and reflex bradycardia (Figure 6).
The Valsalva maneuver. Data from a normal subject, in which the Valsalva maneuver has four phases: (I) there is a brief decrease in heart rate (HR) and increase in systolic blood pressure (BP) due to aortic compression; (II) BP first decreases and then increases; (III) in this phase, due to the release of strain, consecutive declines in BP and increases in HR are observed, preceding a BP overshoot due to persistent sympathetic activity, together with normalization of venous return (IV). The increased BP mediates baroreflex-induced bradycardia and is quantified using the Valsalva ratio, the ratio of the highest HR (II) to the lowest HR (IV). Results depend on the position, age and gender of the subject, as well as the duration and intensity of expiratory pressure. In patients with autonomic dysfunction, there is typically a loss of both BP overshoot and reflex bradycardia. VM: Valsalva maneuver. Adapted from Xavier et al.74
The cold pressor test assesses sympathetic activation mediated by nociceptors, which is observed mainly through BP changes when the hand is immersed up to the wrist in ice-cold water at 4°C. This test, which is predominantly sympathetic, differs from the cold face test, in which the application of a cold stimulus to the face stimulates the trigeminal nerve and elicits bradycardia, and is related to the diving reflex. The cold pressor test, the mental stress test and the handgrip test are the provocative maneuvers mainly used for sympathetic evaluation.
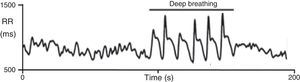
On deep metronomic breathing, autonomic function is assessed with the patient breathing metronomically at a rate of six cycles/min for three minutes, which maximizes respiratory sinus arrhythmia (Figure 7). Changes in HR with deep breathing are a parameter of parasympathetic cardiac control.11,14 The subject's position and body weight, rate and depth of breathing, hypocapnia, sympathetic activity, and use of salicylates and other drugs influence HR variability during deep breathing.
Heart rate response to deep breathing. A normal response showing the imprint of respiration in the heart rate recording during deep breathing. Adapted from Ducla-Soares et al.73
The Ewing battery uses the 30:15 ratio together with blood pressure evaluation in order to assess cardiovascular responses to an active orthostatic challenge. The 30:15 ratio is calculated as the shortest RR interval around the 15th beat divided by the longest RR interval around the 30th beat after standing. Simultaneously with changes in HR there is a physiological decrease in BP. However, if this fall in systolic BP is at least 20 mmHg or in diastolic BP at least 10 mmHg within 3 min of standing, the BP changes are defined as orthostatic hypotension, a sign of cardiovascular autonomic failure.
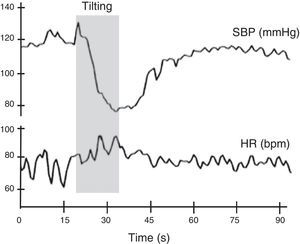
Autonomic evaluation of active standing can be complemented by the head-up tilt test. In theory, this detects the hemodynamic modifications elicited by baroreceptor reflex activation without the interference of the muscular pump of the legs. However, in practice this rarely occurs, as subjects usually develop an alerting reaction when they see the bed beginning to tilt, which is superimposed on the changes in BP and HR caused by baroreflex activation. The hemodynamic changes associated with head-up tilt testing are considered to consist of two stages: an initial acute cardiovascular response lasting around 30 s, and a stabilization phase consisting of an adaptation period 1-2 min after orthostasis followed by a late response to prolonged orthostasis lasting more than 5 min11,14 (Figure 8).
Normal heart rate and blood pressure responses to head-up tilt testing. In this test the subject lies on a tilt test table, which is then tilted upward at 60°-70° after a resting period of at least 5 min. Patients with different degrees of cardiovascular autonomic impairment may show delayed adaptation to the orthostatic challenge or may even be unable to adapt and have a syncopal event. HR: heart rate; SBP: systolic blood pressure. Adapted from Ducla-Soares et al.73
Among the methods for measuring patients’ sympathetic nervous system activity are tests that assess individual sympathetic nervous outflows, such as microneurography and measurement of norepinephrine (NE) spillover to plasma from the sympathetic nerves of individual organs.75,76 Alternatively, overall sympathetic activity can be assessed by analysis of plasma or urine catecholamine concentrations.77,78
Microneurography provides separate recordings of sympathetic nerve activity (SNA) traffic in muscle (MSNA) and skin (SSNA). MSNA reflects the vasoconstrictor signal to the skeletal muscle vasculature. It is highly sensitive to BP changes and is regulated by both arterial and cardiopulmonary reflexes. These reflexes do not affect SSNA. SSNA reflects vasomotor neural traffic to skin blood vessels with almost no sudomotor activity. The two recordings (MSNA and SSNA) differ significantly with regard to morphology. Studies have shown that measurement of sympathetic nerve activity from peripheral nerves is safe, accurate and reproducible. Furthermore, it has been shown that recordings from one limb can be reliably assumed to reflect recordings of sympathetic nerve activity to the muscle vascular bed throughout the body. The method's quantitative nature is also a significant advantage.79,80
Assessment of sympathetic activity based on plasma or urine NE concentration has significant limitations, as NE is subject to changeable reuptake dependent on the density of the basilar plexus and blood flow velocity in a specific organ. Moreover, circulating NE represents only a small fraction (5-10%) of the quantity of the neurotransmitter secreted from nerve terminals.80 Measurement of plasma NE is, however, an improvement over assessment of urine epinephrine, NE and their precursors and metabolites, which was traditionally used to assess ANS tone.79,80
The NE spillover rate has advantages over the above-mentioned methods, since it assesses NE release from specific target organs. The NE radiolabeled method is based on intravenous infusion of small amounts of tritiated NE; tissue clearance of this substance is then subtracted from plasma NE values, and the remainder is a marker of spillover of the neurotransmitter from neuroeffector junctions. In steady-state conditions this spillover reflects the secretion of NE from sympathetic nerve terminals.79,80 Invasive techniques measure total body and regional NE spillover in the heart, splanchnic and renal circulations, and the brain.81
Various methods are used for experimental quantification of sympathetic activity in animals.75,82,83 Direct recordings of SNA (e.g. renal or lumbar) are commonly obtained in animals by the surgical implantation of recording electrodes into the appropriate sympathetic fibers.84
Evaluation of baroreflex functionBaroreceptor function is one of the most important mechanisms regulating moment-to-moment BP. It can be assessed by baroreflex sensitivity (BRS) tests that relate changes in heart period to a BP change. BRS can be assessed under dynamic or steady-state conditions, using physiological or pharmacological approaches. The most common methods include vasoactive drugs (Oxford method), the Valsalva maneuver, the neck chamber technique and analysis of spontaneous BP and HR fluctuations. The Oxford method uses phenylephrine (an alpha-1 adrenergic receptor agonist) to induce a rapid increase in BP (15-40 mmHg) together with HR changes. Modifications of the Oxford method assess BRS through sequential injections of depressor and pressor drugs. There is some controversy with phenylephrine concerning the selectivity of the reflex arc target, since other reflex arcs, particularly the arterial chemoreceptor and the pulmonary mechano- and chemoreceptors, can also be activated. Applying negative or positive pressure to the neck selectively activates carotid baroreceptors and can act as an excitatory or inhibitory stimulus depending on whether positive or negative pressure is applied.
Computer-based techniques (Table 3) assess BRS by correlating spontaneous fluctuations of BP with consecutive HR changes. These computational methods can be divided into time domain, frequency domain and model-based approaches. Time (sequence) and spectral techniques have proven reliability and have become a standard tool in many autonomic testing devices. BRS can be determined by the sequential method.85 This method searches ramps of BP and RR. A ramp defines a variation of at least 1 mmHg and 4 ms between adjacent values of BP and RR, respectively. This concept can only be applied to three or more cardiac cycles varying monotonically, either increasing or decreasing. When a BP ramp occurs at the same time as an RR ramp, a BRS event is identified. BRS can be determined by the mean BRS slope: ΔRRI(ms)/ΔSBP(mmHg), where RRI is RR interval.86 A steeper slope indicates high BRS, while a shallower slope indicates lower sensitivity. The baroreflex effectiveness index (BEI) is the ratio between the total number of BRS events and total number of pressure ramps, increasing or decreasing, for a given period. BEI is an indicator of the effectiveness of baroreceptor-mediated cardiac regulation.
Summary of time, frequency and modeling methods of baroreflex sensitivity assessment.
| Method | Brief description | |
|---|---|---|
| Time domain | Sequences technique108,109 | BRS as the mean of the slopes between SBP and RR values in each identified baroreflex sequence, considering SBP with a 1-beat lag |
| Dual sequence method110 | Equivalent to the sequences technique, identifying baroreflex sequences considering SBP and RR with a shift of up to 3 beats | |
| xBRS111 | BRS as the slope between SBP and RR values over 10-s windows choosing the shift (up to 5 beats) that maximizes SBP and RR cross-correlation. SBP and RR series resampled at 1 Hz | |
| Events technique112 | BRS as one global slope between SBP and RR values in all identified baroreflex events, considering SBP with a 1-beat lag with respect to RR | |
| Frequency domain | Transfer function113 | BRS as the mean magnitude of the transfer function between SBP and RR in the LF band |
| Alpha technique114 | BRS as the square root of the ratio between RR and SBP powers in the LF band | |
| Model-based | Closed-loop bivariate115 | Quantification of feedback and feedforward SBP and RR pathways, assuming a closed-loop SBP and RR system |
| Closed-loop trivariate116 | Quantification of feedback and feedforward SBP and RR pathways, considering two-way pathways between SBP, RR and respiration | |
| xAR117 | Quantification of feedback and feedforward SBP and RR pathways considering respiration as an exogenous input in the SBP and RR loop and assuming a closed-loop SBP and RR system | |
| Causal analysis118 | Quantification of BRS using an exogenous input model to divide RR variability into SBP-related and -unrelated parts |
BRS: baroreflex sensitivity; LF: low-frequency; SBP: systolic blood pressure.
The fact that the rhythm of physiological signals is not entirely regular has raised the possibility of extracting an autonomic signature from these fluctuations using signal-processing techniques. Extremely complex neural mechanisms are responsible for these fluctuations, based mainly on interactions between the sympathetic and parasympathetic nervous system. They therefore represent a rich source of information that can provide considerable insight into the mechanisms of cardiovascular control.87–93 Cardiovascular signals, in particular HR, are most commonly used. However, as in any biological assessment in which the environment affects the result, standardization is a problem, mainly because most autonomic evaluation is performed without a profound knowledge of methods or physiology, which can lead to confounding data and misinterpretation of physiological phenomena. Nonetheless, signal processing methods, when correctly used, are an important tool for identifying autonomic markers and for improving treatment and patient follow-up.
Signal processing can be applied in at least three domains – time, frequency and time-scale – which individually or together can identify different pathological response profiles, such as delays in adaptive responses to provocative maneuvers, dyssynergy between BP and HR responses, and/or exaggerated responses such as orthostatic hypotension, postural orthostatic tachycardia and syncope.94
In particular, fast Fourier transform (FFT) and autoregressive spectral analysis applied to HR and BP signals have made an important contribution to autonomic evaluation.87,94–97 FFT, using sinus functions of different frequencies and amplitudes, decomposes the signals to produce a power spectrum in which for human subjects three major frequency ranges can be recognized: very low frequency (VLF; <0.04 Hz), low frequency (LF; 0.04-0.15 Hz) and high frequency (HF; 0.15-0.4 Hz).98 The VLF band is believed to be related to non-neural factors, such as temperature and hormones.99 The HF band is dominated by the parasympathetic system,98,100 while the LF band is believed to be mediated by both cardiac sympathetic and parasympathetic nervous outflows.
Guzzetti et al. reported that patients with essential hypertension are characterized by greater LF power and smaller HF power of RR interval variability during supine rest compared with normotensive subjects.101 They also reported that the powers showed a smaller increase and decrease, respectively, during passive tilting. These observations were interpreted as indicating that cardiac sympathetic tone is increased and cardiac vagal tone and modulation are decreased in essential hypertension, a conclusion that is in agreement with previous studies in which autonomic cardiac modulation was investigated by different techniques.102,103 FFT analysis, however, has important limitations, as it requires a stationary signal and a long period of data collection, of at least 5 min. Also, it cannot locate and follow changes in a frequency over time.
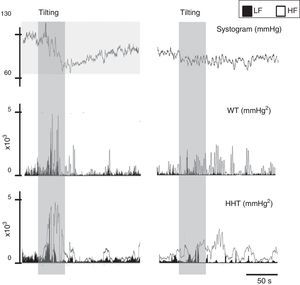
To overcome some of these limitations, like its application to nonstationary and nonlinear signals, a wavelet-based methodology has been proposed to determine the evolution over time of LF and HF frequencies.73 Wavelet analysis is a linear non-stationary representation of signals in the time and frequency domains in which the original signal is decomposed in a shifted version of a basic function, called the mother wavelet, with a different base scale. A mother wavelet function is a non-periodic, oscillatory function that begins and ends at zero in the time domain.104 However, although a good alternative to FFT, wavelets lack resolution, particularly at low frequencies (Figure 9). Wavelet coherence can also be used to analyze the degree of autonomic remodeling in patients under specific therapeutic schemes (Figure 10).
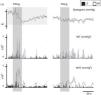
Signal processing of biological signals. Left: data from a normal subject; right: data from a patient with paroxysmal atrial fibrillation. The same signal, the systogram obtained from systolic blood pressure, has been treated with wavelets (Db12) and the Hilbert-Huang transform. The differences in the autonomic output of the two individuals can be clearly distinguished. Note that this example is merely illustrative, as the tachogram obtained from the RR interval for both patients is not shown. HF: high frequency; HHT: Hilbert-Huang transform; LF: low frequency; WT: wavelets.
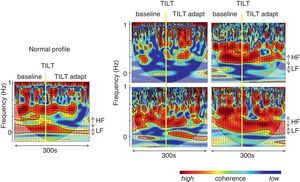
Autonomic analysis tools used to show reverse autonomic modulation in patients with reflex syncope. Left: changes in wavelet coherence evoked by a tilt maneuver in a normal subject. After tilting (vertical line) there is a fall in coherence, which reaches its minimum value approximately 20 s after tilting, recovering later to a significantly lower value than baseline; right: modification of coherence of heart rate and systolic blood pressure variability during a tilt training program designed to induce autonomic remodeling in patients with reflex syncope (A: baseline conditions before training program; B, C and D: 1st, 4th and 9th tilt-training sessions, respectively). The increase in coherence over the course of the training sessions, associated with increased baroreceptor remodeling, is reflected in improvement in band organization together with more intense orange/red color. These changes are more clearly seen after tilting (vertical line). Adapted from Laranjo et al.107
The Hilbert transform is a linear operator able to determine the instantaneous frequency of a signal, corresponding to the convolution of the input signal with the kernel. In order for amplitude, frequency and phase to have physiological application, the signal to be transformed must have an instantaneous null DC component.105 Recently, Huang proposed fulfilling this condition through empirical mode decomposition (EMD), which can be applied to non-linear and non-stationary processes. The combination of the Hilbert transform with EMD results in what is known as the Hilbert-Huang transform.
ConclusionThe ANS has moved towards center stage in cardiovascular medicine. Dysregulation of this system contributes to cardiovascular disease, including hypertension, atrial fibrillation and other cardiac arrhythmias, ischemic heart disease, obesity, diabetes, atherosclerosis, sleep apnea, metabolic syndrome and congestive heart failure, and is often associated with a more severe disease burden. However, there are serious gaps in our understanding of ANS function, and treatment options targeting the ANS are still in their infancy. It is important to begin by performing a thorough assessment of the ANS as it relates to the cardiovascular system. There are as yet no gold standard tests for autonomic testing and differences in test performances are an obstacle to comparing scientific and clinical data acquired in different laboratories. With this review, we hope to provide valuable help by focusing on standard tests for diagnosis of cardiovascular autonomic dysfunction.
Conflicts of interestThe authors have no conflicts of interest to declare.