Recurrent ventricular tachycardia (VT) episodes have a negative impact on the clinical outcome of implantable cardioverter-defibrillator (ICD) patients. Modification of the arrhythmogenic substrate has been used as a promising approach for treating recurrent VTs. However, there are limited data on long-term follow-up.
AimTo analyze long-term results of VT substrate-based ablation using high-density mapping in patients with severe left ventricular (LV) dysfunction and recurrent appropriate ICD therapy.
MethodsWe analyzed 20 patients (15 men, 55% with non-ischemic cardiomyopathy, age 58±15 years, LV ejection fraction 32±5%) and repeated appropriate shocks or arrhythmic storm (>2 shocks/24 h) despite antiarrhythmic drug therapy and optimal heart failure medication. All patients underwent ventricular programmed stimulation (600 ms/S3) to document VT. A sinus rhythm (SR) voltage map was created with a three-dimensional electroanatomic mapping system (CARTO, Biosense Webster, CA) using a PentaRay® high-density mapping catheter (Biosense Webster, CA) to delineate areas of scarred myocardium (ventricular bipolar voltage ≤0.5 mV – dense scar; 0.5-1.5 mV – border zone; ≥1.5 mV – healthy tissue) and to provide high-resolution electrophysiological mapping. Substrate modification included elimination of local abnormal ventricular activities (LAVAs) during SR (fractionated, split, low-amplitude/long-lasting, late potentials, pre-systolic), and linear ablation to obtain scar homogenization and dechanneling. Pace-mapping techniques were used when capture was possible. The LV approach was retrograde in nine cases, transseptal in five and epi-endocardial in four. In two patients ablation was performed inside the right ventricle.
ResultsLAVAs and scar areas were modified in all patients. Mean procedure duration was 149 min (105-220 min), with radiofrequency ranging from 18 to 70 min (mean 33 min) and mean fluoroscopy time of 15 min. Non-inducibility was achieved in 75% of cases (in four patients with hemodynamic deterioration and an LV assist device, VT inducibility was not performed). There were two cases of pericardial tamponade, drained successfully. During a follow-up of 50±24 months, 65% had no VT recurrences. Among the seven patients with recurrences, three underwent redo ablation and four, with fewer VT episodes, received appropriate ICD therapy. There were five hospital readmissions due to heart failure decompensation, one patient died in the first week after unsuccessful ablation of a VT storm and three died (stroke and pneumonia) >1 year after ablation.
ConclusionCatheter ablation based on substrate modification is feasible and safe in patients with frequent VTs and severe LV dysfunction. This approach may be of clinical relevance, with potential long-term benefits in reducing VT burden.
Os episódios de taquicardia ventricular (TV) recorrente têm um impacto negativo na evolução clínica de doentes (D) com cardioversor-desfibrilhador implantável (CDI). A modificação do substrato arritmogénico tem sido usada como uma abordagem promissora para o tratamento de TV recorrente. No entanto, são limitados os dados sobre o seguimento em longo prazo.
ObjetivoAnalisar os resultados em longo prazo da ablação de TV baseada na modificação do substrato, com recurso a mapeamento de alta densidade em D com disfunção ventricular esquerda (VE) grave e terapia recorrente apropriada via CDI.
Métodos20D (15 homens, miocardiopatia não isquémica 55%, 58±15 anos, fração de ejeção do VE 32±5%) e choques apropriados recorrentes ou tempestade arrítmica (> 2 choques/24h) apesar de terapêutica medicamentosa antiarrítmica e otimização da medicação para insuficiência cardíaca. Todos os D foram submetidos a estimulação ventricular programada (600ms/S3) para documentação da TV. Foi criado um mapa de voltagem em ritmo sinusal (RS) com sistema de mapeamento eletroanatómico 3D (CARTO, Biosense Webster, CA), usando um cateter de mapeamento de alta densidade PentaRay® (Biosense Webster, CA) para delinear áreas de miocárdio com cicatriz (voltagem bipolar ventricular ≤0,5mV - cicatriz densa; 0,5-1,5mV - zona de contorno; ≥ 1,5mV - tecido saudável) e fornecer mapeamento eletrofisiológico de alta resolução. A modificação do substrato incluiu a eliminação de locais com atividade eletrofisiológica ventricular anormal (LAVA: eletrogramas fracionados, com duplo potencial, de baixa amplitude/longa duração, potenciais tardios, pré-sistólicos) durante RS e ablação linear para obter a homogeneização das cicatrizes, com eliminação de canais de condução lenta. A técnica de pace-mapping foi usada sempre que a captura local era possível. A abordagem do VE foi retrógrada em nove casos, transeptal em cinco e endoepicárdica em quatro casos. Em 2D a ablação endocárdica foi realizada no ventrículo direito.
ResultadosAs áreas de LAVA e cicatriz foram modificadas em todos os D. A duração média do procedimento foi de 149min (105-220min), com tempo de radiofrequência variando de 18 a 70min (média 33min) e tempo médio de fluoroscopia de 15min. A não inducibilidade foi obtida em 75% dos casos (em 4P - deterioração hemodinâmica/dispositivo de assistência VE - a inducibilidade de TV não foi realizada). Foram drenados dois tamponamentos pericárdicos com sucesso. Durante um follow-up de 50±24 meses, 65% não tiveram recorrências de TV. Entre os 7D com recorrências, três foram submetidos a «redo» da ablação e 4D, com menos episódios de TV, receberam terapia apropriada do CDI. Houve cinco reinternamentos hospitalares devido à descompensação de insuficiência cardíaca, 1D faleceu na 1.ª semana após ablação sem sucesso, devido a tempestade arrítmica e ocorreram 3 óbitos > 1 ano após a ablação (acidente vascular cerebral e pneumonia).
ConclusãoA ablação por cateter baseada na modificação do substrato é exequível, eficaz e segura em D com episódios recorrentes de TV e disfunção VE grave. Esta abordagem pode ter relevância clínica, com benefícios potenciais em longo prazo na redução da carga de TV.
In patients with structural heart disease, recurrent ventricular tachycardia (VT) episodes have a negative impact on clinical outcome, even in those who have received an implantable cardioverter-defibrillator (ICD).1 Elimination of arrhythmic reentry circuits represents a difficult challenge, mainly due to the induction of intolerable VTs, frequently with multiple electrocardiographic morphologies, requiring rapid interruption.2 In recent years, substrate-based ablation has been used as an effective strategy for treating recurrent VTs, particularly after myocardial infarction.3 The precise localization of scars and abnormal potentials can be critical for ablation success. The use of three-dimensional (3D) electroanatomic mapping systems with high-density multipolar catheters, combined with imaging techniques like magnetic resonance (MRI) or computed tomography (CT), enables more precise acquisition of electrograms (EGMs), providing valuable anatomic and functional information that may contribute to the safety and success of VT ablation. Few studies have reported long-term results after substrate-based VT ablation in patients with severe left ventricular (LV) dysfunction. Moreover, despite the association of catheter VT ablation with a significant reduction in appropriate ICD therapies, VT storm and cardiac hospitalizations in patients with ischemic cardiomyopathy,4 preventive VT ablation strategies before ICD shocks seem not to affect hard clinical outcomes.5 We therefore sought to assess long-term outcomes of a substrate-based ablation strategy using high-density mapping in patients with severe LV dysfunction and recurrent appropriate ICD therapy.
MethodsPatient populationThe subject cohort included 20 consecutive patients from a single center, with LV systolic dysfunction (due to ischemic or to non-ischemic cardiomyopathy) and an ICD implanted for primary prevention, with documented repeated appropriate shocks or arrhythmic storm (>2 shocks/24 h) despite antiarrhythmic drug therapy and optimal heart failure medication. Four patients with non-ischemic cardiomyopathy underwent cardiac MRI before the procedure (not included in the study protocol). All patients provided written informed consent to undergo electrophysiological study (EPS) and catheter ablation to perform substrate modification of VT.
Electrophysiological studyEPS and ablation were conducted in a fasting state, under conscious sedation except in four cases in which EPS was performed after induction of general anesthesia following LV assist device placement. Continuous blood pressure monitoring and digital pulse oximetry were performed. ICD therapies were inactivated. Programmed stimulation was performed (with up to two extrastimuli decremented to ventricular refractoriness and rapid pacing) using a multipolar steerable 6-F diagnostic catheter placed in the right ventricular apex, in order to document VT morphology, confirm hemodynamic instability during VT, and test for VT inducibility. Hemodynamic instability was defined as a decrease of <80 mmHg in systolic blood pressure for >10 s. VT was terminated by overdrive pacing or by electrical cardioversion.
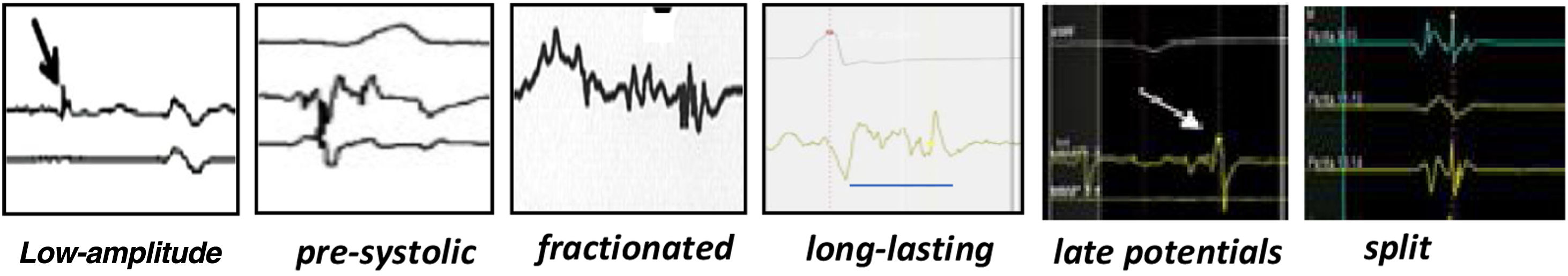
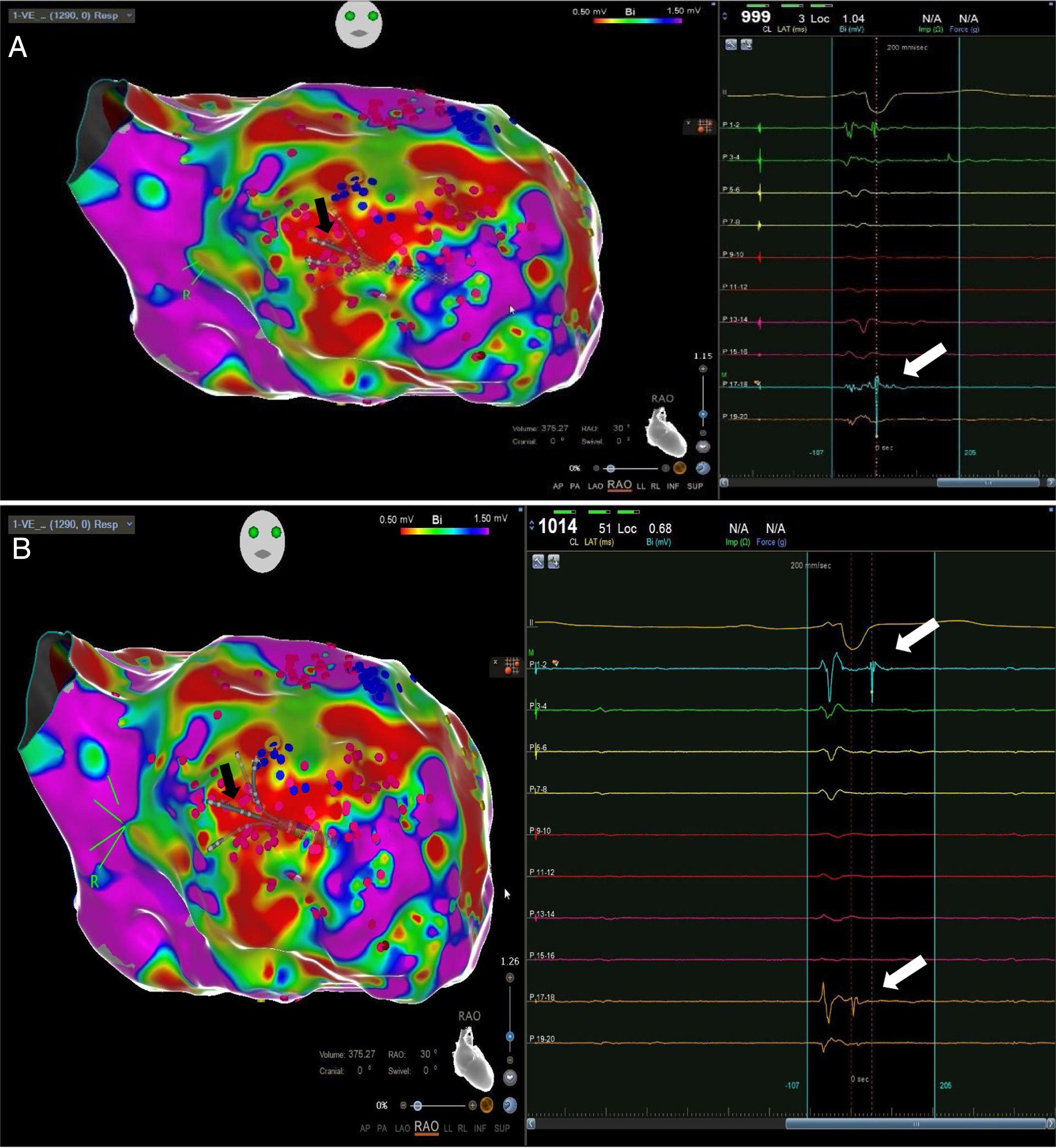
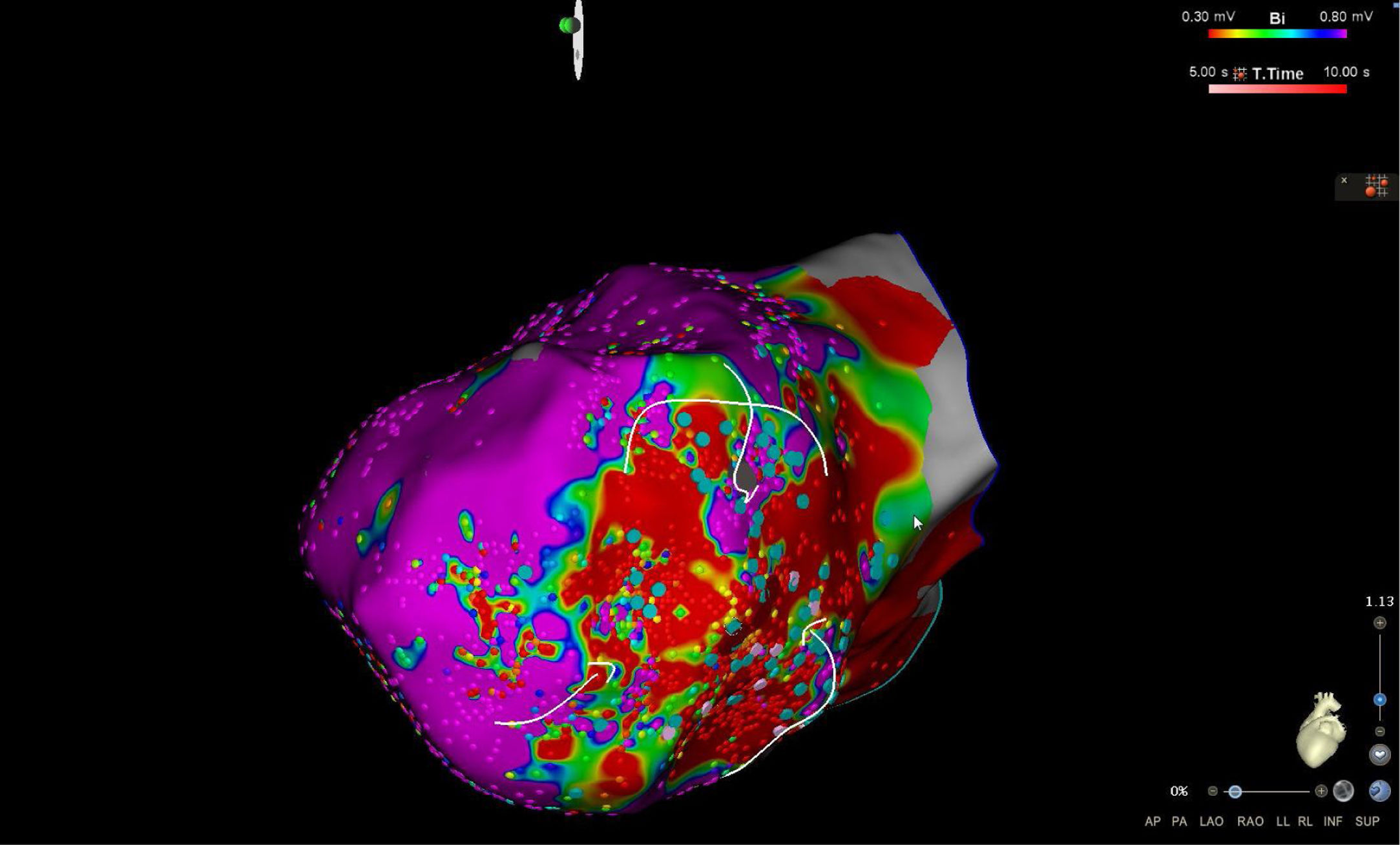
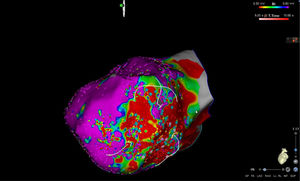
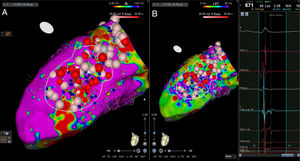
High-density electroanatomic mappingA sinus rhythm (SR) voltage map was created with a 3D electroanatomic mapping system (CARTO, Biosense Webster, CA), using a high-density mapping catheter (PentaRay®, Biosense Webster, CA) to delineate areas of scarred myocardium (ventricular bipolar voltage ≤0.5 mV – dense scar; 0.5-1.5 mV – border zone; ≥1.5 mV – healthy tissue)6 and to provide high-resolution detailed electrophysiological substrate mapping. For epicardial mapping, the normal voltage amplitude was set above 1.0 mV. Bipolar EGMs were filtered from 10 to 400 Hz. Local abnormal ventricular activities (LAVAs)6,7 during SR (fractionated, split, low-amplitude/long-lasting, late potentials, pre-systolic), and zones of slow conduction with continuous multicomponent and delayed EGMs within myocardium scars (conducting channels), were marked accordingly on the map (Figures 1 and 2). Conduction channels were also visualized in the electroanatomic mapping by adjusting the color threshold on the voltage map (Figure 3). Image integration with previously acquired contrast-enhanced cardiac CT (64-slice scanner), when available, was performed using the CartoMerge image integration module (Biosense Webster, CA).
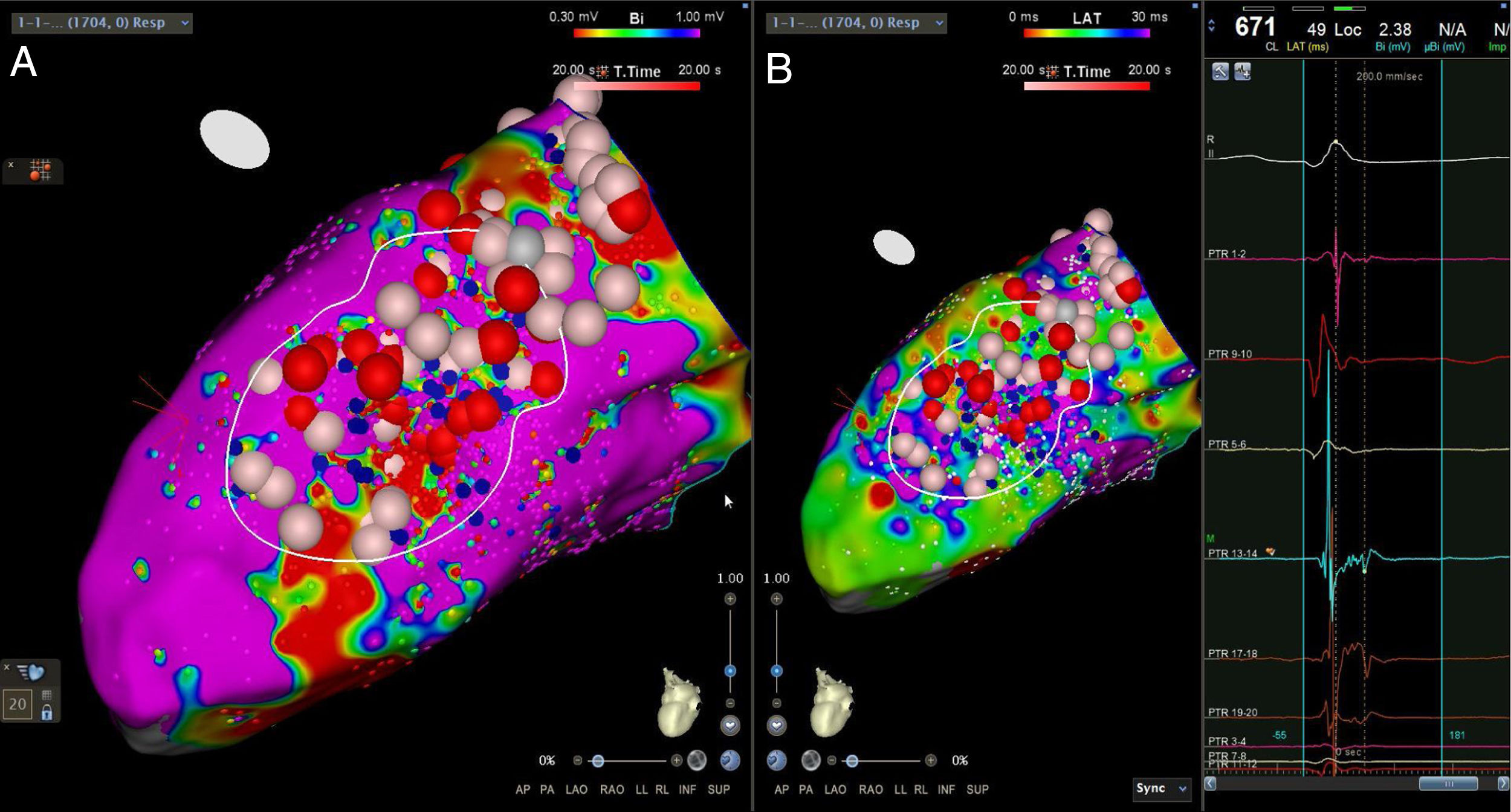
The LV approach was retrograde in nine cases, transseptal in five (Brockenbrough needle; Medtronic Inc, Minneapolis, MN and the Agilis NxT steerable introducer, Abbott) and epi-endocardial in four (when endocardial ablation alone was unsuccessful). In two patients radiofrequency (RF) ablation was performed inside the right ventricle. Pace-mapping was performed at sites within channels when capture was possible. Substrate modification included elimination of LAVAs and linear ablation with sequential point lesions to obtain scar homogenization and scar dechanneling (Figure 4). These lesions were extended until pacing could not capture the tissue. RF energy applications were tagged for display on the CARTO system. To avoid potentially adverse effects on ventricular function, RF lesions were applied exclusively in myocardium with abnormal voltage.
RF ablation was performed using a saline-irrigated ablation catheter (ThermoCool, Biosense Webster, CA) in 60-s applications, under power control of 30-40 W, infused with irrigation at 2 ml/min during catheter manipulation or 15-17 ml/min during RF delivery, with impedance monitoring and a temperature limit of 42°C. Activated clotting time was maintained over 220 s. After substrate modification by elimination of all identified LAVAs, complete scar homogenization with linear lesions, and failure to pace inside the scar area, ventricular programmed stimulation was repeated to test for VT inducibility. Acute success was defined as no induction of sustained monomorphic VT or induction of a morphologically indeterminate VT or ventricular fibrillation.
After ablation, patients were followed by remote monitoring, maintaining in-hospital ICD interrogations once a year and after VT episodes. The primary endpoint was recurrence of sustained VT. All patients were discharged under antiarrhythmic therapy with amiodarone or dofetilide and a beta-blocker.
Statistical analysisBaseline parameters are expressed as mean ± SD or proportion for continuous and categorical variables, respectively. Comparisons were performed using the Student's t test to compare paired continuous variables, the chi-square test (or Fisher's exact test, where applicable), or the Mann-Whitney U test, as appropriate. A p value of <0.05 was considered significant. The statistical analysis was carried out using GraphPad Prism 8.4.3 (GraphPad Software).
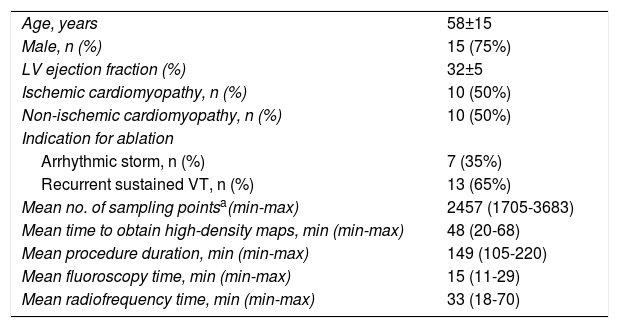
ResultsBaseline characteristics of the study populationThe mean age of the 20 patients was 58±15 years, 75% were male, and mean LV ejection fraction was 32±5% (range, 25-35%). There were 10 patients (50%) with previous myocardial infarction (anterior/septal in four and inferior/lateral in six, two of them with extension to the right ventricle), and 10 patients with non-ischemic cardiomyopathy (50%). The four cases with previous MRI had late enhancement in the interventricular septum (n=2), LV apex (n=1) and right outflow tract (n=1). No patients had previous VT ablation. The baseline characteristics of the study population are summarized in Table 1.
Baseline clinical characteristics and main procedural data.
| Age, years | 58±15 |
| Male, n (%) | 15 (75%) |
| LV ejection fraction (%) | 32±5 |
| Ischemic cardiomyopathy, n (%) | 10 (50%) |
| Non-ischemic cardiomyopathy, n (%) | 10 (50%) |
| Indication for ablation | |
| Arrhythmic storm, n (%) | 7 (35%) |
| Recurrent sustained VT, n (%) | 13 (65%) |
| Mean no. of sampling pointsa(min-max) | 2457 (1705-3683) |
| Mean time to obtain high-density maps, min (min-max) | 48 (20-68) |
| Mean procedure duration, min (min-max) | 149 (105-220) |
| Mean fluoroscopy time, min (min-max) | 15 (11-29) |
| Mean radiofrequency time, min (min-max) | 33 (18-70) |
LV: left ventricular; max: maximum; min: minimum; VT: ventricular tachycardia.
VT was induced in all patients, ranging from one to six morphologies, with a cycle length between 280 and 600 ms. All cases with sustained VT presented marked hypotension followed by successful interruption with bursts or external DC shocks. Patients underwent substrate-based ablation with detailed maps of the LV. The approach was retrograde in nine cases, transseptal in five and epi-endocardial in four. In two cases (inducible VT with left bundle branch block morphology and left superior axis; one with ischemic cardiomyopathy, one with non-ischemic cardiomyopathy) with septal scar areas and a perfect matching of the VT morphology during pace-mapping, the right ventricle was also mapped.
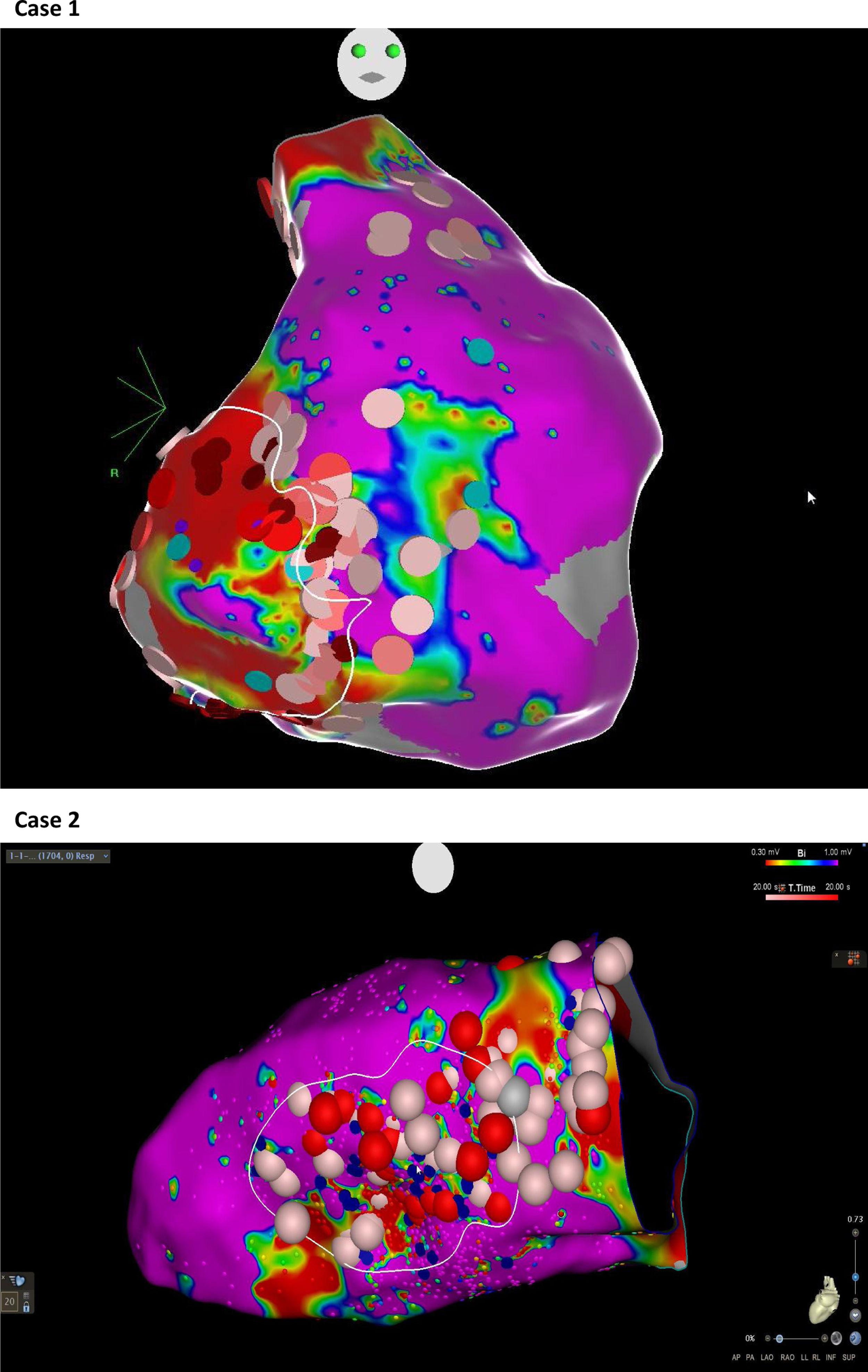
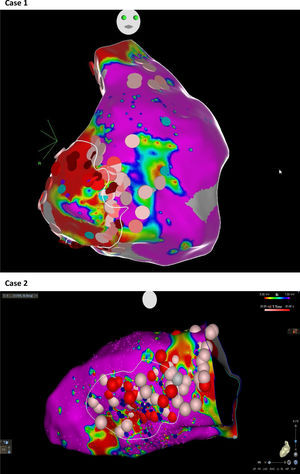
Abnormal electrograms and scar areas were identified and modified in all patients. In 50% of the cases, a >90% match (for the first induced monomorphic VT), consistent with a location close to the exit site of the tachycardia circuit, was obtained with pace-mapping at sites within channels. Figure 5 shows examples of substrate-based ablation in patients with previous anterior myocardial infarction (case 1) and non-ischemic cardiomyopathy with areas of scar (case 2). The mean duration of the procedure was 149 min (105-220 min), with RF applications ranging from 18 to 70 min (mean 33 min) and a mean fluoroscopy time of 15 min (11-29 min).
Procedure results and ablation outcomesIn four patients, due to hemodynamic deterioration and use of an LV assist device (extracorporeal membrane oxygenation [ECMO] in two cases, intra-aortic balloon counterpulsation in two cases), VT inducibility was not attempted. VT inducibility was tested in 16 patients. Non-inducibility was achieved in 75% of cases. In the other four cases, no further RF applications were attempted. There were two cases of pericardial tamponade, drained successfully.
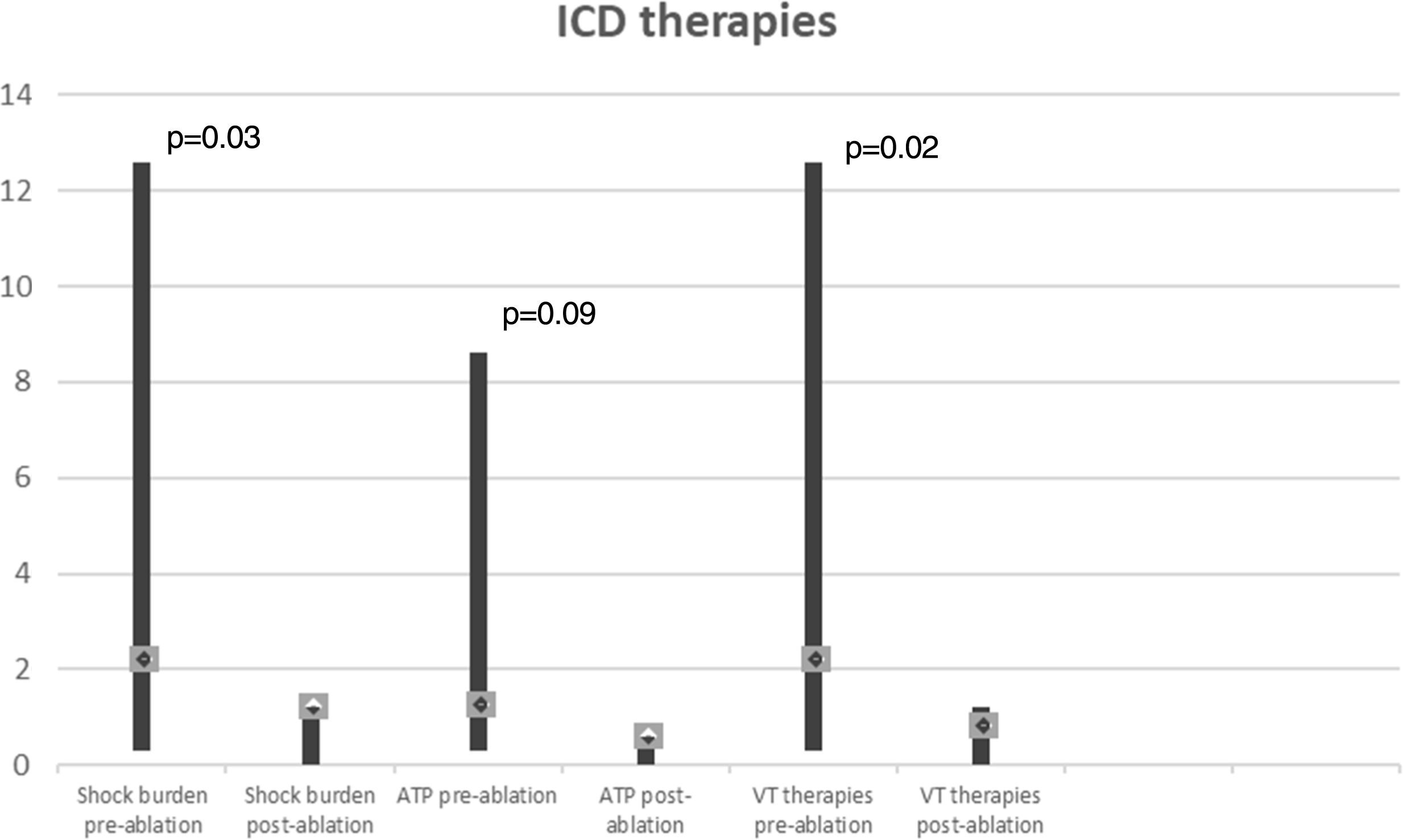
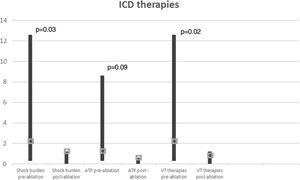
In a mean of 50±24 months of follow-up, there were no VT recurrences with ICD therapies in 65% of cases. Among the seven patients with recurrences (two not tested for VT induction and two with VT inducible after substrate modification), one patient, with ECMO, died with incessant VT developing refractory pump failure 72 hours after ablation, three underwent a redo ablation and three, with fewer VT episodes, received appropriate ICD therapy (1-3 episodes in total). The VT recurrence rate did not differ between patients with and without VT inducibility after substrate-based ablation (p=0.6). Comparison of VT burden treated by ICD in the year before ablation and at one-year follow-up showed a significant reduction in the frequency of VT episodes (Figure 6): mean ICD shock frequency was reduced from 12.8±21.0 (range 3-86) in the year prior to ablation to 0.9±1.2 (range 0-4) over the first year of follow-up (p=0.03).
There were five hospital readmissions due to heart failure decompensation, without documented VT. Three patients died more than one year after ablation due to pneumonia (n=2) or stroke (n=1).
DiscussionMain findingsIn this study, we report our experience using a high-density mapping catheter to perform substrate-based VT ablation, without mapping the arrhythmia circuit during VT, in patients with an ICD for primary prevention and severe LV dysfunction, presenting with VT storm or recurrent VT episodes and failure of antiarrhythmic medication. We showed that this group of patients, with VT intolerance, may benefit from a standard simplified, and probably faster, method of ablation, based on substrate modification with RF after defining low-voltage areas, LAVAs and conduction channels (inside or on the border of scar tissue). In a recent analysis of the VISTA multicenter randomized trial, Di Basie et al. showed that, despite longer RF time, a substrate-based approach in SR was performed with a shorter procedural time.8
In the present study, the mean duration of the procedure was around 150 min, including VT inducibility before and after ablation, high-density mapping to identify scar areas and LAVAs, pace-mapping attempts and RF delivery (mean time 33 min), which is a slightly shorter duration than seen in previous studies with a VT substrate-based approach.8–10 In the VISTA trial, with extensive substrate-based ablation targeting all LAVAs, the reported mean RF time was 68 min, twice the RF time in this study.8
To date, no studies have compared different substrate ablation strategies in terms of efficacy, safety, and procedural requirements. Decreasing the procedure time and complexity of VT ablation procedures while maintaining effectiveness has been considered a marker of hospital survival and lower risk of complications.11 In a previous study, VT induction and mapping prolonged the procedure and increased radiation exposure and the need for electrical cardioversion without significantly improving ablation results and long-term outcomes in comparison with substrate ablation alone.9 Berruezo et al. demonstrated that testing inducibility with VT mapping and ablation before substrate modification did not contribute to the acute results of the procedure or arrhythmic outcomes.13
Ablation performed using a substrate-based approach requires the precise and accurate definition of the border zones and identification of channels and isthmuses. High-density multipolar catheters used in contemporary mapping systems can obtain accurate maps with rapid automated continuous acquisition and reduced fluoroscopy. We were able to create detailed voltage maps (with a mean of 2457 collecting points) and identify LAVAs, within and on the edge of the scar in all patients, in less than 60 min in most cases. Although the procedure is time-consuming, following this high-resolution strategy also enabled us to locate VT conduction channels and define areas for ablation lines and spot RF applications. Several studies have shown that ablation of VT substrates, mostly based on scar homogenization, dechanneling and elimination of LAVAs, significantly reduces VT recurrence.9,10,12 In our experience, this substrate modification is feasible, with an acceptable rate of complications (two cases of cardiac tamponade successfully managed by pericardiocentesis) and significant long-term benefits regarding VT burden, with 65% of patients without recurrence and a significant reduction in the number of VT episodes treated by ICD (Figure 6).
Ventricular tachycardia mapping and substrate modificationSubstrate-based strategies include targeting LAVAs, including fractionated, split, low-amplitude/long-lasting, pre-systolic and late potentials, and scar dechanneling and homogenization. Various authors have adopted different substrate modification approaches without mapping VT circuits, using VT inducibility only after substrate-based ablation,12,13 and showed that more than half of the patients had no inducible VT after substrate modification with significant freedom from VT during a two-year follow-up, patients non-inducible after substrate ablation having better outcomes. In our study, non-inducibility was obtained in 75% of patients (12 out of 16 tested cases), with VT recurrence occurring in 25% (vs. 50% in the four cases with VT inducibility after ablation). Non-inducibility after VT ablation has been associated with better outcomes,14,15 whereas inducibility of VT after substrate ablation probably reflects the presence of unmodified or inaccessible circuits. Although our results are in accordance with previous studies reporting around 30% of non-inducibility after VT substrate ablation, it is known that the degree of sedation, the induction protocol and the stimulation site may influence inducibility of VT. Therefore, the absence of inducibility does not mean that VT will not recur, and thus it should not be considered to be consistently associated with better outcomes.
Our experience also shows that LV assist devices may permit mapping and modification of the VT arrhythmic substrate in hemodynamically unstable patients. However, there is no evidence that mechanical circulatory support improves acute or follow-up results.16
LimitationsThis was a single-center study, with a limited sample size and with no randomized comparison of ablation strategies or ischemic cardiomyopathy versus non-ischemic cardiomyopathy patients. Although we consider it a limitation, this is a prospective observational report of consecutive patients admitted with arrhythmic storm or recurrent VT episodes treated by ICD, severe LV dysfunction, with a long follow-up after a uniform protocol of high-density mapping and substrate ablation. Substrate mapping was performed only during SR in all patients, without analyzing the effect of different activation wavefronts (right or left ventricular pacing) on the arrhythmic substrate. It has been suggested that the rhythm and direction of activation wavefronts at the time of mapping may alter the identification of LAVAs and therefore influence substrate-based ablation.17 Finally, the small sample size and the induction of multiple VT morphologies makes it difficult to validate the role of non-inducibility in VT recurrence and survival in this group of patients.
ConclusionsCatheter ablation of VT based on substrate modification, with complete LAVA elimination, scar homogenization and dechanneling, guided by high-density mapping, is feasible and safe in patients with severe LV dysfunction and unmappable recurrent VT. This approach may be of clinical relevance, with potential benefits in improving long-term freedom from VT, with most patients having their VT completely controlled and the other cases showing a reduction in appropriate ICD therapies. These data support the concept of a substrate-based ablation strategy in the setting of multiple sustained VT episodes and structural heart disease. Further randomized studies are warranted to assess whether substrate modification using this technique is associated with a better outcome.
Conflicts of interestThe authors have no conflicts of interest to declare.