Mortality in patients with end-stage renal disease is higher than in the general population. This is linked to traditional and non-traditional cardiovascular (CV) risk factors, as well as with risk factors associated with end-stage renal disease itself. The aim of this study is to identify CV risk markers in patients beginning peritoneal dialysis (PD) and their association with CV events and CV mortality.
MethodsThis was a retrospective cohort study of 112 incident PD patients, in which demographic, clinical and laboratory parameters, valvular calcifications, types of PD solutions, hospitalizations, CV events and death were analyzed. Occurrence of CV events or death due to a CV event after PD initiation was defined as the primary endpoint. The use of icodextrin solution was taken as a marker of hypervolemia.
ResultsMean age was 53.7±16.1 years. Patients were treated with PD for 29.3±17.4 months. Eighteen patients (16.1%) had valvular calcifications at baseline, 15 patients (13.4%) had major CV events and 11 patients (9.8%) died from CV-related causes. Cox proportional hazards analysis of CV events or CV-related mortality revealed that mitral calcification, use of icodextrin solution and low albumin were independent predictors of CV events or mortality.
ConclusionsTraditional CV risk factors appear to have little impact on CV complications in PD patients. Nevertheless, hypervolemia, hypoalbuminemia and mitral calcifications were independent predictors of CV events or mortality in this group of patients.
A taxa de mortalidade nos doentes com doença renal crónica terminal é significativamente mais elevada em comparação à população geral. Para isso contribuem fatores de risco cardiovascular (CV) clássicos e fatores de risco associados à própria doença renal crónica. Com este estudo pretendeu-se identificar marcadores de risco cardiovascular em doentes incidentes em diálise peritoneal (DP), e a sua associação a eventos cardiovasculares ou mortalidade cardiovascular.
MétodosEstudo de coorte retrospetivo de 112 doentes incidentes em DP, onde os dados demográficos, clínicos e laboratoriais, calcificações valvulares, tipo de soluções de DP, hospitalizações, eventos cardiovasculares e morte de causa cardiovascular após o início de DP foram considerados como outcomes primários. A utilização de solução de icodextrina foi usada como um marcador de hipervolemia.
ResultadosA idade média foi de 53,7 ± 16,1 anos. Os doentes foram tratados com DP durante 29,3 ± 17,4 meses; 18 doentes (16,1%) apresentavam calcificações valvulares à data de início da técnica. Quinze doentes (13,4%) tiveram eventos CV major e 11 doentes (9,8%) morreram na sequência de eventos CV. A análise estatística por regressão de Cox mostrou que a calcificação mitral, o uso da solução de icodextrina e a hipoalbuminemia foram preditores independentes de eventos CV ou mortalidade CV.
ConclusõesOs fatores tradicionais de risco CV aparentam ter pouco impacto no desenvolvimento das complicações CV dos doentes em DP. Porém, a hipervolemia, a hipoalbuminemia e a calcificação mitral foram preditores independentes de eventos CV ou mortalidade neste grupo de doentes.
bone alkaline phosphatase
confidence interval
C-reactive protein
cardiovascular
estimated glomerular filtration rate
end-stage renal disease
fluid overload
hemodialysis
hazard ratio
intact parathyroid hormone
normalized protein catabolic rate
peritoneal dialysis
receiver operating characteristic
Chronic kidney disease is associated with an increased risk of stroke.1 Mortality in patients with end-stage renal disease (ESRD) is much higher than in the general population in spite of advances in dialysis treatment. Reasons for this higher incidence include greater risk of cardiovascular (CV) disease and associated higher comorbidity and mortality in ESRD patients, including peritoneal dialysis (PD) patients.2,3 In addition, CV mortality is up to 100 times higher in these patients than in the general same-age population, especially in younger groups.4,5 Worsening renal function also has an unquestionably negative impact on prognosis in patients with acute heart failure.6
Traditional risk factors for CV disease such as older age, diabetes, hypertension and hyperlipidemia, non-traditional CV risk factors related to PD therapy such as decreased residual renal function,7 ultrafiltration failure, peritoneal protein loss and use of glucose-based solutions,8 and also novel risk factors such as chronic inflammation,9,10 have all been established as CV risk factors in these patients.11–13
Additionally, ESRD-related risk factors, such as fluid overload, anemia, mineral metabolism disorders and poor nutritional state play an important role in the prognosis of PD patients.
Although the effects of various CV risk factors on morbidity and mortality in PD patients are recognized, CV disease is still frequently under-diagnosed and under-treated in these patients. Up until recently, predictors of CV risk in PD patients have not been extensively investigated in incident PD patients.
The aim of this study is to identify potential attributes or characteristics that affect the occurrence of fatal and non-fatal cardiovascular events in patients beginning PD.
MethodsThis is a retrospective cohort study performed at a single PD unit, based on 112 adult incident patients admitted to the PD program over a five-year period (2010-2014).
For the patient survival analysis, subjects were censored after the first CV event, at transplantation, transfer to hemodialysis (HD), transfer to another PD center or when completing the follow-up period. Survival status was censored on November 30, 2015.
Patients were excluded if they were under 18 years of age at the time of PD initiation, had acute infection or were clinically unstable, had previous renal graft failure, or had a life expectancy shorter than six months.
Demographic characteristics (age, gender), etiology of chronic kidney disease, previous comorbidities, and laboratory results (albumin, C-reactive protein, total cholesterol and triglycerides) were recorded at baseline. The presence of valvular calcification (mitral, aortic or both) at the start of dialysis was also assessed by Doppler echocardiography.
Anthropometric data and other laboratory measurements (adjusted calcium level, phosphorus, intact parathyroid hormone [iPTH], and bone alkaline phosphatase [BAP]) as well as adequacy of PD and nutrition parameters were obtained at the beginning of PD and also at the last assessment.
Peritoneal function was assessed through the peritoneal equilibration test (PET). Peritoneal transport characteristics were identified using dialysate/plasma creatinine reference values after a PET and PD adequacy was assessed using weekly Kt/V, peritoneal ultrafiltration, residual creatinine clearance and normalized protein catabolic rate (nPCR).
Patients who were treated with icodextrin solution were recorded.
Patients were classified as having a major CV event if the main cause of death or hospitalization was acute coronary syndrome, ischemic heart disease, congestive heart failure or stroke. Occurrence of a CV event or death due to a CV event after PD initiation was defined as the primary endpoint. Hypervolemia was diagnosed on the basis of the presence of specific criteria: dyspnea, raised jugular venous pressure and basal crepitations; radiographic evidence of pulmonary venous congestion or interstitial edema; and resolution of symptoms, signs and radiographic changes with icodextrin exchanges. The use of icodextrin solution was thus taken as a marker of hypervolemia.
The study was approved by the institutional ethics committee and all participants gave their written consent.
Statistical analysisCategorical variables were described as number or percentage of relative frequencies and quantitative variables as mean ± standard deviation (SD) for continuous normally distributed variables.
Differences between clinical data were assessed by the Student's t test for paired samples for continuous variables with normal distribution and the paired Wilcoxon test for continuous data with non-normal distribution. Survival curves were computed by the Kaplan-Meier method. Unadjusted and adjusted hazard ratios (HRs) for all-cause and CV events or mortality were estimated by multivariate regression analysis with Cox proportional regression and reported with 95% confidence intervals (CIs). A p value of <0.05 was considered statistically significant. To identify patients at highest risk for the study endpoint, albumin values and the corresponding endpoint rates were related via receiver operating characteristic (ROC) curves.
All statistical tests were performed using the Statistical Package for the Social Sciences (SPSS), version 14.0 (SPSS Inc., Chicago, IL, USA).
ResultsTable 1 presents clinical and biochemical parameters for the study group at PD initiation. All 112 patients studied were dialyzed using bicarbonate-buffered PD solution, and 48 (42.95%) patients were treated with icodextrin solutions. Mean age was 53.7±16.1 (range 20-85) years; 65.2% were male and 37.8% had diabetes. At baseline, 107 patients (95.6%) were treated with antihypertensive drugs (mean 3; standard deviation 1.1) and 20 patients (17.9%) were prescribed statins due to hyperlipidemia. Body mass index at PD initiation was 25.74±4.66 kg/m2. Patients were treated with PD for 29.3±17.4 months. The etiologies of ESRD are described in Table 1.
Clinical and biochemical parameters for the study group at initiation of peritoneal dialysis.
| Patients (n=112) | |
| Male (%) | 65.2 |
| Diabetes (%) | 37.8 |
| Age (years) | 53.7±16.1 (20-85) |
| CKD etiology | |
| Diabetic nephropathy | 35 (31.3%) |
| Unknown | 22 (19.6%) |
| CGN | 17 (15.2%) |
| Nephroangiosclerosis | 12 (10.7%) |
| ADPKD | 8 (7.1%) |
| Chronic pyelonephritis | 7 (6.3%) |
| Other etiologies | 11 (9.8%) |
| BMI (kg/m2) | 25.74±4.66 |
| Comorbidities | |
| Ischemic cardiopathy | 20 (17.9) |
| Stroke | 11 (13.6) |
| Heart failure | 14 (12.5) |
| CAPD/APD (n/%) | 76 (67.9)/36 (32.1) |
| Icodextrin (n/%) | 48 (42.9) |
| CRP (mg/dl) | 1.17±1 |
| Albumin (g/dl) | 3.43±0.58 |
| Phosphorus >5.5 mg/dl (%) | 26.4 |
| iPTH >500 pg/ml (%) | 31.2 |
| Total cholesterol (mg/dl) | 201±46.1 |
| Triglycerides (mg/dl) | 149±43.3 |
| BAP <18 UI/l (%) | 63.4 |
| nPCR <1 g/kg/day (%) | 50 |
ADPKD: autosomal dominant polycystic kidney disease; APD: automated peritoneal dialysis; BAP: bone alkaline phosphatase; BMI: body mass index; CAPD: continuous ambulatory peritoneal dialysis; CGN: chronic glomerulonephritis; CKD: chronic kidney disease; CRP: C-reactive protein; iPTH: intact parathyroid hormone; nPCR: normalized protein catabolic rate.
During the PD follow-up period, no changes were observed between initial and final assessments in the following results: adjusted calcium: 9.05 vs. 9.03 mg/dl (p=NS), nPCR: 0.95 vs. 0.92 g/kg/day (p=NS) and peritoneal ultrafiltration: 685.4 vs. 720.1 ml/day (p=NS). However, iPTH (p=0.001), BAP (p=0.011) and phosphorus levels (p<0.001) were higher in the final assessments (Table 2). Residual renal function (eGFR: 6.76 vs. 4.49 ml/min/1.73 m2, p<0.001) and dialysis adequacy (Kt/v: 2.48 vs. 2.26, p=0.017) deteriorated over time.
Changes in laboratory values at initiation and at last follow-up of peritoneal dialysis treatment.
| PD initiation | Last follow-up | p | |
|---|---|---|---|
| Calcium (mg/dl) | 9.05 | 9.03 | NS |
| nPCR (mg/kg/day) | 0.95 | 0.92 | NS |
| iPTH (pg/ml) | 469.97 | 624.69 | 0.001 |
| BAP (UI/l) | 16.18 | 21.03 | 0.011 |
| Phosphorus (mg/dl) | 5.03 | 5.62 | <0.001 |
| eGFR (ml/min/1.73 m2) | 6.76 | 4.49 | <0.001 |
BAP: bone alkaline phosphatase; eGFR: estimated glomerular filtration rate; iPTH: intact parathyroid hormone; nPCR: normalized protein catabolic rate; PD: peritoneal dialysis.
Valvular calcifications were assessed at baseline. Ninety patients (80.4%) were assessed at PD initiation by Doppler echocardiography, of whom 18 (16.1%) had valvular calcifications (two patients had exclusively aortic calcification, four had mitral calcification only and 12 had both). Besides these, no significant valvular dysfunction (stenosis or regurgitation) was found in these patients.
During the five-year observation period, 15 patients (13.4%) had major CV events and 11 (9.8%) died from CV-related causes. Time until the first CV event or death from CV-related causes was 26.3±17.8 months.
Twelve patients (10.7%) received a kidney allograft and 33 (29.4%) were transferred to hemodialysis.
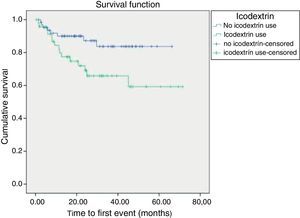
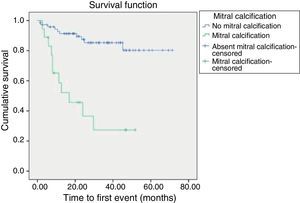
Cox proportional hazards analysis of CV events or mortality from CV-related causes revealed that mitral calcification (HR: 3.25, 95% CI: 1.3-8.3, p=0.001), use of icodextrin solution (HR: 3.8, 95% CI: 1.13-12.8, p=0.003) and albumin <3.35 g/dl (HR: 2.84, 95% CI: 0.15-7.0, p=0.024) were independent predictors of CV events or mortality, in a model adjusted to diabetes and age.
There was no increased risk of CV events or mortality with different PD solutions (calcium levels of 1.25 mmol/l vs. 1.75 mmol/l; p=NS). Also, elevated serum phosphorus, elevated calcium-phosphorus product and elevated C-reactive protein could not predict CV events or mortality (p=NS).
Kaplan-Meier survival curves revealed that time until first event (defined as a major CV event or death from CV-related causes) was shorter in patients treated with icodextrin (p=0.025) and in patients with mitral calcification (p<0.001) (Figures 1 and 2).
Cardiovascular disease is common in renal failure and the causes of CV events in these patients are usually multifactorial. Their manifestations are multiple and heterogeneous and most aspects are not well studied in PD patients. Most information on CV complications in ESRD patients covers both HD and PD patients and few studies consider only incident patients. Additionally, published randomized controlled trials solely on PD patients had small population samples, inadequate study power and short follow-up periods, and rarely examined hard primary outcome measures such as mortality and CV events.
In our study, we determined that certain non-traditional risk factors were important predictors of major CV events and/or death in PD patients.
In our five-year observation period, 26 patients had CV events: 11 patients died (9.8%) from CV-related causes and 15 (13.4%) had major CV events. Some biases could explain the paucity of major CV events: the young age of the cohort and the study's inclusion criteria (considering only incident patients without acute infection or clinical instability, previous renal graft failure or life expectancy shorter than six months). Besides, fluid overload (FO) is one of the most studied conditions in ESRD patients. FO is frequently associated with CV complications,14,15 inflammation16 and mortality17,18 in these patients, although few studies have shown a direct relation between clinically assessed FO and outcome in PD patients. A recent study from China19 demonstrated that overhydrated patients had significantly increased all-cause mortality and a trend for increased CV mortality and technique failure. Moreover, patients with FO had significantly more cardiac and cerebrovascular events.
Furthermore, PD patients experiencing difficulty in maintaining euvolemia due to insufficient peritoneal ultrafiltration are usually treated with icodextrin solution once daily as an alternative to hypertonic glucose PD solutions for long dwells. Use of icodextrin was accordingly taken in our study as a marker of hypervolemia.
In our population, 48 patients (42.9%) were treated with icodextrin and these patients had a higher incidence of CV events or death (HR: 3.8, 95% CI: 1.13-12.8, p=0.003). Icodextrin was mainly prescribed for patients with significant FO, in line with the addition of icodextrin to PD treatment in order to increase peritoneal ultrafiltration volume and control FO. So, as the use of icodextrin was heavily associated with FO, it can be used as a marker of vascular fragility, identifying PD patients at risk of CV events or death. Nevertheless, icodextrin was not associated with drop-out from the PD program (p=NS).
Furthermore, a study from Turkey16 showed that FO was significantly correlated with malnutrition, inflammation and atherosclerosis. A PD patient developing malnutrition may gradually accumulate extracellular water to balance loss of body cell mass or body fat mass. Additionally, the existence of malnutrition, inflammation and atherosclerosis (MIA) syndrome is now established. Inflammation promotes neoangiogenesis, generation of profibrotic factors, progressive increase in peritoneal permeability, loss of ultrafiltration and more fluid accumulation.20,21 Inflammation also contributes to the development of endothelial dysfunction, atherosclerosis and vascular calcification through the secretion of acute phase proteins and cytokines, complement activation and immune cell recruitment.20 Inflammation is also accompanied by malnutrition, hypoalbuminemia and reduction in oncotic pressure. In our study, albumin <3.35 g/dl (HR: 2.84, 95% CI: 0.15-7.0, p=0.024) was an independent predictor of CV events or mortality.
The third independent predictor of major CV event or death was mitral calcification (HR: 3.25, 95% CI: 1.3-8.3, p=0.001).
Surprisingly, only 14.4% of patients who were assessed by Doppler echocardiography at PD initiation had valvular calcifications (aortic, mitral or both), a significantly smaller percentage of patients than in recently published studies.22–24 A Spanish study22 established the prevalence of valvular calcification at the start of dialysis and the relationship between valvular calcification and the presentation of composite endpoints of acute myocardial infarction, stroke or death from CV causes in the follow-up of incident dialysis patients. Vascular calcification had a prevalence of 50% at the beginning of dialysis, which differs significantly from our results. Once again, this may be due not only to the younger age of our patients (10 years younger comparing the mean age of both study populations), but also to the fact that our patients were exclusively treated by PD. Patients from the other series22 were treated by both PD and HD, which makes comparisons difficult.
Other studies in the literature on valvular calcification in dialysis patients only refer to prevalent patients, describing high prevalence, between 30% and 70% in PD and HD patients.
Nevertheless, both studies obtained a similar result: valvular calcification was an independent predictor of CV events or death related to major CV events. In other studies on prevalent HD25 and PD26 patients, valvular calcification was an independent predictor of all-cause mortality, and is accepted as a marker of CV disease.
Our study has limitations. It was performed in a single center and included a relatively small population, and valvular calcifications, CV events and deaths occurred in a relatively small number of patients.
Nevertheless, predictors of CV events or death were consistent with the published literature. This report is also one of the few in which only incident and exclusively PD patients were studied. The encouraging results (few CV events and few deaths) were probably related to the young age of this patient group. Randomized controlled trials are needed to confirm these results, in order to monitor traditional and non-traditional risk factors in PD patients. These results could provide significant prognostic information that will be useful in clinical practice for patient management, since these predictors are signaling variables of frail patients at the beginning of PD, indicating the need to adjust dialysate solutions to maintain euvolemia.
ConclusionsTraditional CV risk factors appear to have little impact on CV complications in PD patients. Nevertheless, hypervolemia, hypoalbuminemia and mitral calcifications were independent predictors of CV events or mortality in this group of patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.