Neurohormonal blockade (NB)/modulation is the combination of two renin-angiotensin-aldosterone system inhibitors (RAASi) with a beta blocker. It is the core therapy for heart failure with reduced ejection fraction (HFrEF). While improving long term prognosis, it also induces hyperkalemia (serum K+ >5.0 mEq/L) due to RAASi effects. This may cause lethal arrhythmias and increase mortality in the short term. Thus, hyperkalemia frequently leads to withholding or reducing the intensity of neurohormonal blockade/modulation, which is associated with worsening long term prognosis. We assessed the relevance of hyperkalemia as a limiting factor of neurohormonal blockade/modulation in real life clinical conditions.
MethodsWe reviewed the medical records of HFrEF patients attending a HF clinic at a tertiary Portuguese hospital during 2018 (n=240). The number of patients not tolerating maximal neurohormonal blockade/modulation due to hyperkalemia was determined. The incidence and characteristics of hyperkalemia episodes were also assessed.
ResultsOnly six patients (3%) achieved maximal doses of neurohormonal blockade/modulation. Hyperkalemia was the limiting factor in 48 (20%) patients. A total of 185 hyperkalemia episodes occurred in 100 (42%) patients. Forty-five (24%) episodes were moderate or severe (serum K+ >5.5 mEq/L). In these HFrEF patients, the co-existence of hypertension, diabetes or renal failure was associated with the occurrence of hyperkalemia.
ConclusionsIn daily clinical practice, hyperkalemia is frequent and limits neurohormonal blockade/modulation by leading to the withholding or reducing of the intensity of RAAS inhibition. Considering the negative prognostic impact associated with sub-optimal neurohormonal blockade/modulation, addressing hyperkalemia is an important issue when treating HFrEF patients.
O bloqueio/modulação neuro-hormonal (BMNH) é a combinação de dois inibidores (i) do sistema Renina-Angiotensina-Aldosterona (RAAS) com um betabloqueador. Como principal terapêutica na insuficiência cardíaca (IC) com fração de ejeção reduzida (FEr), melhora o prognóstico em longo prazo, porém induz hipercaliemia (K+ sérico >5,0 mEq/L), pode causar arritmias letais e aumentar a mortalidade em curto prazo. A hipercaliemia evita ou reduz frequentemente a intensidade do BMNH, o que está associado a pior prognóstico em longo prazo. Avaliámos a relevância da hipercaliemia como fator limitante do BMNH em condições clínicas da vida real.
MétodosReviram-se os registos médicos dos doentes com ICFEr num hospital terciário português em 2018 (n=240). Determinou-se o número de doentes sem tolerância de BMNH máximo por hipercaliemia e avaliou-se as incidências/características dos episódios de hipercaliemia.
ResultadosApenas seis doentes (3%) atingiram doses máximas de BMNH. A hipercaliemia foi o fator limitante em 48 (20%) doentes. Ocorreram 185 episódios de hipercaliemia em 100 (42%) doentes. Destes, 45 (24%) foram moderados ou graves (K+ sérico >5,5 mEq/L). A coexistência de hipertensão, diabetes ou insuficiência renal foi associada à ocorrência de hipercaliemia.
ConclusõesNa prática clínica, a hipercaliemia é frequente e limita o BMNH, evitando ou reduzindo a intensidade da inibição de RAAS. Considerando o impacto prognóstico negativo associado ao BMNH subótimo, a abordagem da hipercaliemia é uma questão importante no tratamento de doentes com ICFEr.
Heart failure (HF) is a growing public health concern that affects 26 million people worldwide.1 Its prevalence is predicted to increase by 25% in the next decade.2 HF is the main cause of hospitalizations >65 years of age in Europe and in the United States of America (USA).3 HF hospitalizations have high associated morbidity and mortality4 and are the main driver of HF-associated costs.5,6 In the USA and Europe, HF has the greatest economic impact of all diseases.1 In Portugal, HF is estimated to represent 2.6% of the total health budget,7 and the loss of disability-adjusted life years is predicted to increase 27.9% by 2036.8 HF mortality is higher than that associated with the most common forms of cancer.9
Heart failure with reduced ejection fraction (HFrEF) guidelines recommend timely institution of triple neurohormonal blockade/modulation. This is the combination of two renin-angiotensin-aldosterone system inhibitors (RAASi) with a beta blocker (BB): an angiotensin-converting enzyme inhibitor (ACEi), or an angiotensin receptor blocker (ARB), or an angiotensin receptor-neprilysin inhibitor (ARNI), associated with a mineralocorticoid receptor antagonist (MRA) and a BB.10
The use of maximal doses of all these drugs is strongly recommended, as studies have shown increased mortality and morbidity among patients who do not reach target doses.11–14 However, this is often hampered by adverse pharmacological effects, such as hyperkalemia, which can occur with all RAASi. According to Juurlink et al.,15 after the publication of the RALES study,16 an increase in spirolactone prescription and hyperkalemia-associated morbidity and mortality was observed. While mild hyperkalemia (serum K+ 5.1–5.5 mEq/L) is generally well tolerated, higher levels of serum potassium are associated with an increased risk of potentially lethal arrhythmias.17,18 The European Society of Cardiology (ESC) HFrEF guidelines recommend halving the dose or discontinuation of RAASi in the presence of moderate (serum K+ 5.6–6.0 mEq/L) or severe hyperkalemia (serum K+ >6.0 mEq/L), respectively.10 However, sub-optimal RAASi doses or RAASi discontinuation is, in turn, linked to worse long term HF prognosis. Hence, new potassium-binders, which lower serum potassium concentration, have been developed, to enable RAASi utilization.19–22 Nevertheless, evidence from clinical practice is still limited.
The present study aims to identify the proportion of HFrEF patients, in real life clinical practice, who cannot reach the neurohormonal blockade/modulation strategy target doses due to hyperkalemia. The recognition of this treatment gap clarifies the magnitude of the unmet need of enabling RAASi with potassium-binders in the optimization of HFrEF patients’ daily care.
MethodsDesignThis is a retrospective observational study conducted at an outpatient HF clinic at the Cardiology Department of a Portuguese University Hospital Center.
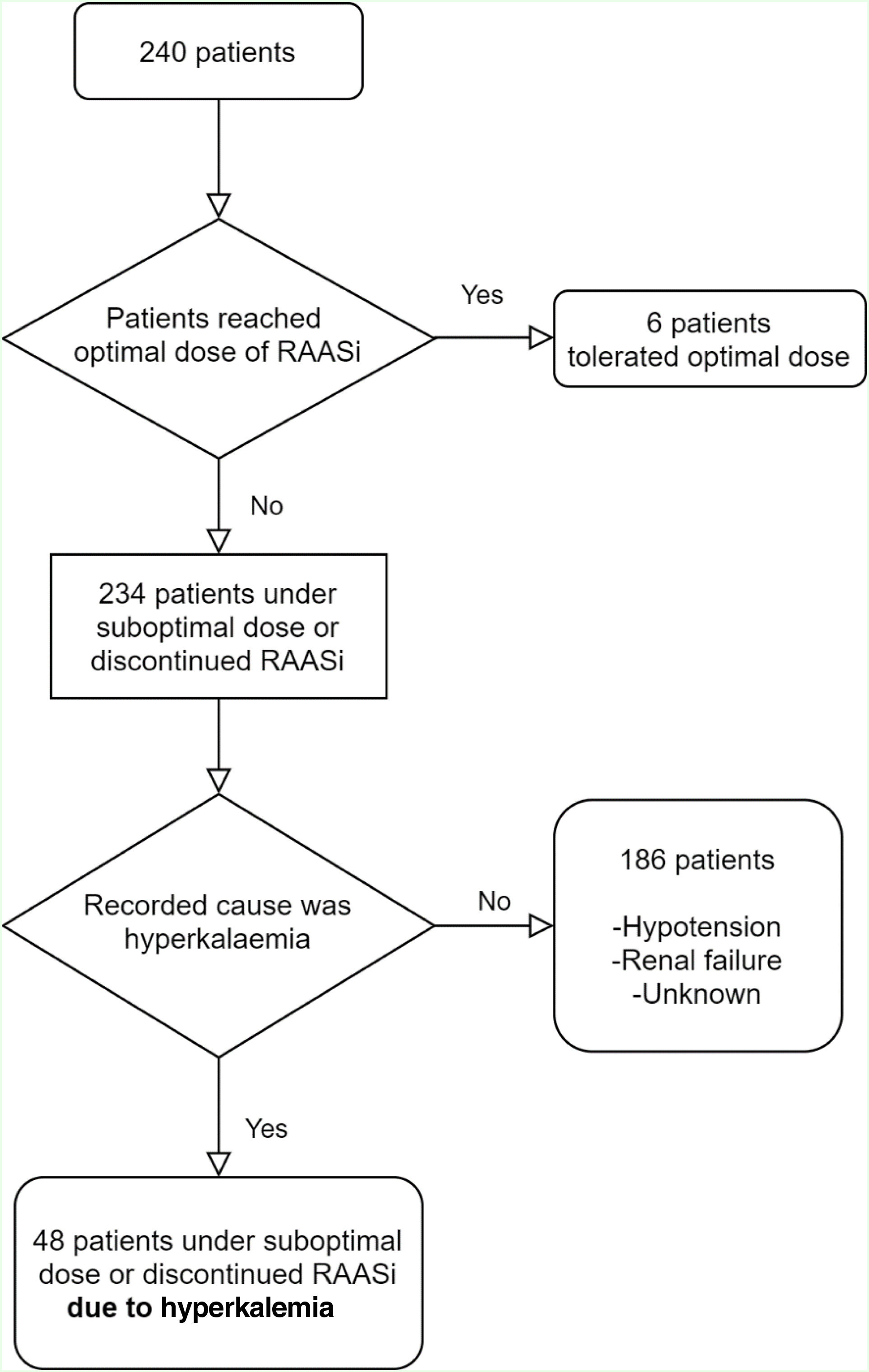
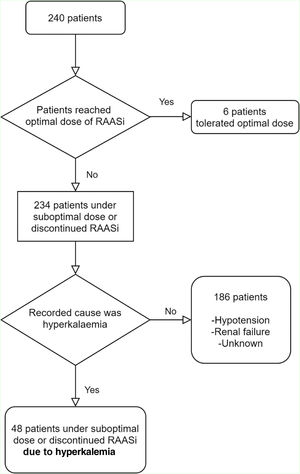
PatientsAll consecutive patients with HFrEF, attending the HF clinic at the university hospital center during 2018 were included (Figure 1).
Inclusion criteria were: age ≥18 years old, HF diagnosed according to the 2016 ESC Heart Failure Guidelines,10 and left ventricular ejection fraction (LVEF) <40%.
No exclusion criteria were applied.
Data collectionFor each patient, the first appointment in 2018 was defined as the index visit in the present study. After inclusion in the study, each patient's medical records from 1 January 2016 to 31 December 2019 were reviewed.
Quantitative and qualitative data were collected from the patients’ electronic health records, including age, gender, HF etiology, New York Heart Association functional class, HF signs and symptoms, echocardiographic data, blood and urine laboratorial data and medication history. Only laboratorial and echocardiographic data preceding medical appointments for a three- and six-month maximum period, respectively, were considered.
The presence of comorbidities, including diabetes, dyslipidemia and hypertension, was recorded. Obesity was defined as a body mass index >30 kg/m2.
All episodes of hyperkalemia for each patient were identified. Renal failure was considered clinically relevant in patients presenting renal failure stage ≥3 (estimated glomerular filtration rate <60 mL/min/1.73 m2 evaluated by the Chronic Kidney Disease Epidemiology Collaboration formula). Hyperkalemia was defined as serum potassium level >5.0 mEq/L, and further divided into mild (5.1–5.5 mEq/L), moderate (5.6–6.0 mEq/L) and severe (>6.0 mEq/L).
Medication records were reviewed at all medical appointments during follow-up. In the case of neurohormonal blockers, the dose was converted into the class's reference drug equivalent: enalapril in the case of ACEis, candesartan in the case of ARBs, bisoprolol in the case of BBs, and spironolactone in the case of MRAs. The target doses for each neuro-hormonal blocker were those mentioned in the 2016 ESC HF guidelines.10 Reasons for drug dose reduction, drug withdrawal or absence of drug up-titration were captured or marked as “unknown” when no information was available.
OutcomeThe primary outcome was the proportion of HFrEF patients not reaching neurohormonal blockade/modulation target doses due to hyperkalemia.
EthicsAll procedures were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The protocol was approved by the Health Ethics Committee of the university hospital center.
Data analysisStatistical analysis and data management were performed with MS Excel and R Studio using R software, version 3.6.2 (R Foundation for Statistical Computing). Continuous variables were expressed as mean and standard deviation when normally distributed, and as median and interquartile range otherwise. Categorical variables were summarized as counts and percentage of total sample for each class. Missing data cases were excluded from the number of cases in each calculation. Chi-squared test was used to assess differences in prevalence of diabetes, hypertension and renal failure among cohorts.
ResultsOf all the patients with an appointment at the outpatient HF Clinic during 2018, 240 met our inclusion criteria. Median LVEF was 29% (interquartile range (IQR): 24–35).
Patients’ characteristics at index visit are listed in Table 1. Mean age was 61±11 years and 173 (72%) were men.
Patient characteristics at the 2018 index visit.
| Age (years), mean±SD | 61.4±10.5 |
| Male gender, n(%) | 173/240 (72.1%) |
| BMI (kg/m2), mean±SD | 28.4±4.7 |
| Ischemicaetiology, n(%) | 95/240 (39.6%) |
| Risk factors, n(%) | |
| Dyslipidemia | 174/240 (72.5%) |
| Hypertension | 117/238 (49.2%) |
| Obesity | 79/218 (36.2%) |
| Ex-smoker | 84/240 (35.0%) |
| Current smoker | 34/240 (14.2%) |
| Diabetes | 81/238 (34.0%) |
| NYHA class, n(%) | |
| I | 52/237 (21.9%) |
| II | 136/237 (57.4%) |
| III | 43/237 (18.1%) |
| IV | 6/237 (2.5%) |
| SBP (mmHg), mean±SD | 117.0±18.8 |
| DBP (mmHg), mean±SD | 67.7±10.7 |
| HR (bpm), mean±SD | 68.4±11.5 |
| BNP (pg/mL), median (IQR) | 146.5 (65.7–378.2) |
| LVEF (%), median (IQR) | 29 (23.5–35.0) |
| Creatinine (mg/dL), median (IQR) | 0.91 (0.78–1.14) |
| CKD Stage, n(%) | |
| 1 | 50/205 (24.4%) |
| 2 | 77/205 (37.6%) |
| 3 | 60/205 (29.3%) |
| 4 | 10/205 (4.9%) |
| 5 | 3/205 (1.5%) |
| Potassium (mEq/L), mean±SD | 4.6±0.6 |
| Medication, n(%) | |
| ACEis | 171/240 (71.2%) |
| ARBs | 34/240 (14.2%) |
| BBs | 228/240 (95.0%) |
| MRAs | 200/240 (83.3%) |
| ARNi | 10/240 (4.2%) |
ACEi: Remove capitals from all that are not proper nouns; ARB: Angiotensin II Receptor Blocker; ARNi: Angiotensin Receptor-Neprilysin inhibitor; BB: Beta-Blocker; BMI: Body Mass Index; BNP: B-type natriuretic peptide; CKD: Chronic Kidney Disease; DBP: Diastolic Blood Pressure; HR: Heart Rate; LVEF: Left Ventricular Ejection Fraction; MRA: Mineralocorticoid Receptor Antagonist; NYHA: New York Heart Association; SBP: Systolic Blood Pressure.
In 95 (40%) patients, HF etiology was ischemic heart disease. Regarding cardiovascular risk factors, 174 (73%) of patients had dyslipidemia, 118 (49%) a history of smoking, 117 (49%) hypertension, 81 (34%) diabetes and 79 (36%) were obese. In 73 (36%) patients, estimated glomerular filtration rate was <60 ml/min/1.73 m2.
Patients were followed for a median of 160 (IQR: 107–182) weeks. Each patient attended a median of 3.1 appointments per year (IQR: 2.4–4.1). All patients had information regarding the prescribed medication.
Only six patients (3%) reached maximal doses of NH blockade/modulation. Forty-eight (20%) patients were under suboptimal RAASi doses or discontinued one or more RAASi specifically due to hyperkalemia: in 12 (5%) patients one or more RAASi was not prescribed and in 36 (15%) RAASi target doses were not reached. Other reasons include hypotension, bradycardia renal failure and a group of unlisted reasons. Tables 2 and 3 show the tolerated doses of RAASi for the entire sample, and for those patients whose therapy was hampered by hyperkalemia, respectively.
Tolerated drug dose level by pharmacological class for all patients (n=240).
| Dose | ACEi | ARB | MRA | ARNi |
|---|---|---|---|---|
| Target dose | 80/192 (41.7%) | 4/36 (11.1%) | 37/219 (16.9%) | 23/97 (23.7%) |
| Suboptimal dose | 92/192 (47.9%) | 29/36 (80.6%) | 164/219 (74.9%) | 70/97 (72.2%) |
| Stopped drug | 20/192 (10.4%) | 3/36 (8.3%) | 18/219 (8.2%) | 4/97 (4.1%) |
ACEi: Angiotensin-Converting Enzyme inhibitor; ARB: Angiotensin II Receptor Blocker; ARNi: Angiotensin Receptor-Neprilysin inhibitor; MRA: Mineralocorticoid Receptor Antagonist.
Tolerated drug dose level by pharmacological class in patients who had their treatment hampered by hyperkalemia (n=48).
| Dose | ACEi | ARB | MRA | ARNi |
|---|---|---|---|---|
| Target dose | 22/40 (55.0%) | 0/5 (0.0%) | 0/41 (0.0%) | 6/23 (26.1%) |
| Suboptimal dose | 15/40 (37.5%) | 5/5 (100.0%) | 30/41 (73.2%) | 17/23 (73.9%) |
| Stopped drug | 3/40 (7.5%) | 0/5 (0.0%) | 11/41 (26.8%) | 0/23 (0.0%) |
ACEi: Angiotensin-Converting Enzyme inhibitor; ARB: Angiotensin II Receptor Blocker; ARNi: Angiotensin Receptor-Neprilysin inhibitor; MRA: Mineralocorticoid receptor antagonist.
Overall, 100 patients (42%) presented at least one episode of hyperkalemia during the follow-up. A total of 185 hyperkalemia episodes across these patients were registered: 140 (76%) mild, 40 (22%) moderate and 5 (3%) severe.
Patients presenting one or more episodes of hyperkalemia during the follow-up were older (mean age 63±10 vs. 60±11, p=0.027), and had higher prevalence of renal failure (44% vs. 29%, p=0.037), hypertension (61% vs. 41%, p=0.004) and diabetes (46% vs. 26%, p=0.003). These results are summarized in Table 4.
Characteristics of patients with no (n=140) vs. one or more hyperkalemia episodes (n=100).
| Variable | Number of hyperkalemia episodes | p-valuea | |
|---|---|---|---|
| None | One or more | ||
| Age (years), mean±SD | 60±11 | 63±10 | 0.027b |
| Male gender, n(%) | 99/140 (70.7%) | 74/100 (74.0%) | 0.679 |
| CKD stage ≥3, n(%) | 33/114 (28.9%) | 40/91 (44.0%) | 0.037 |
| Diabetes, n(%) | 36/139 (25.9%) | 45/99 (45.5%) | 0.003 |
| Hypertension, n (%) | 57/139 (41.0%) | 60/99 (60.6%) | 0.004 |
In HFrEF, maximal RAASi doses are associated with better prognosis compared with submaximal doses.11–14 However, in everyday clinical practice RAASi up-titration to maximal doses encounters several obstacles. Among them are hypotension, renal failure and hyperkalemia.15,17,18 In our study, we found that hyperkalemia was the cause of RAASi under-titration in 20% of our HFrEF patients.
The percentage of our patients not reaching RAASi maximal doses due to hyperkalemia aligns with that described in previous real life studies, in which hyperkalemia was present in 4–21% of cases.23
Our study showed that hyperkalemia is frequent in real life clinical practice. The prevalence of hyperkalemia episodes in 42% of our patients is in line with previous reports.23,24 The fact that 24% of hyperkalemia episodes were moderate or severe is a striking result since they are associated with an increased risk of sudden arrhythmic death.25,26 The association of hyperkalemia with older age, diabetes, chronic kidney disease and hypertension has also been previously described.23,27
Hyperkalemia leads to the downgrade of RAASi therapy, which is associated with worse prognosis and increased hospitalizations11 and costs.28 Thus, an approach that simultaneously prevents hyperkalemia and enables RAASi therapy at full dose is a clear and present unmet need. Our study is a contribution to establishing the dimension of this unmet need in daily practice.
Two new potassium-binding drugs, patiromer and sodium zirconium cyclosilicate, have been studied in the OPAL-HK,19 PEARL-HF,20 HARMONIZE,21 and AMETHYST-DN22 trials. They have recently been approved and shown to be able to normalize elevated K+ levels and prevent recurrent hyperkalemia in patients with diabetes, chronic renal disease, and heart failure. Additionally, data on patiromer demonstrated the RAASi-enabling capacity of this drug in the setting of chronic renal disease, diabetes, and congestive heart failure.19–22 Patiromer maintained normokalemia during one year follow-up.22 Currently, the DIAMOND trial is assessing the effective potential of patiromer in reducing cardiovascular death and hospitalizations by enabling optimal RAASi therapy in patients with HFrEF.29 In daily clinical practice, hyperkalemia is frequent and limits NH blockade/modulation by hampering RAASi therapy. Addressing hyperkalemia and enabling RAASi is an important issue in HFrEF treatment with the potential of reducing morbidity, mortality, and costs.
This study has limitations concerning data collection, as they were retrospectively gathered from medical records which were not designed for clinical research. A more granular registry may allow for a more powerful exploration of hyperkalemia and associated factors in future research.
ConclusionsHyperkalemia is a common side effect of RAASi therapy. It is associated with an increased risk of potentially lethal arrhythmias in patients with HF. This study, conducted under real clinical practice conditions in a Portuguese center, identified the proportion of HFrEF patients who cannot reach RAASi target doses due to hyperkalemia. Our results underline the need for hyperkalemia-controlling strategies to optimize HFrEF patient care.
FundingThis work was supported by Vifor Pharma, Portugal, via an educational grant. Support for third-party medical writing assistance for this manuscript, furnished by Ana Macedo, MD, PhD of Keypoint, was provided by Vifor Pharma.
Conflicts of interestThe authors have no conflicts of interest to declare.