To report the hemodynamic profile and short- and medium-term outcomes of Freedom Solo and Solo Smart stentless aortic valves implanted at our center.
MethodsBetween 2009 and 2015, all patients undergoing aortic valve replacement using Solo stentless valves at our center were enrolled. Clinical and echocardiographic follow-up was carried out six months postoperatively. Survival and major events, including structural valve deterioration and non-structural valve dysfunction, endocarditis, reoperation and stroke, were assessed through medical records or telephone interview with the referring cardiologist up to November 2015 (mean and maximum follow-up 39±22 and 78 months, respectively).
ResultsPatients’ (n=345) mean age was 72±8 years, 52% were female and median euroSCORE II was 2.7 (1.5-4.7). There was no intraoperative mortality and in-hospital mortality was 2.6%. Postoperatively, mean transvalvular gradient was 11.9±4.5 mmHg and effective orifice area was 1.9±0.5 cm2. Patient-prosthesis mismatch occurred in 14% but was severe in only one patient. Cumulative survival at six years was 72%. Six patients were reoperated: three due to endocarditis, two for structural prosthesis deterioration and one because of periprosthetic fistula. Five patients suffered stroke, three had medically-treated endocarditis and one had structural valve deterioration but was not considered suitable for reoperation. None of the remainder had structural valve deterioration or non-structural valve dysfunction.
ConclusionsSolo stentless aortic valves are safe to implant, with promising clinical outcomes in short- and medium-term assessment. Moreover, they show an excellent hemodynamic performance: low transvalvular gradients, large effective orifice areas and low incidence of patient-prosthesis mismatch.
Descrever o perfil hemodinâmico e resultados clínicos a curto e médio prazo das biopróteses aórticas stentless Freedom SOLO e SOLO Smart implantadas no nosso Centro.
MétodosForam incluídos todos os doentes submetidos a substituição valvular aórtica por biopróteses stentless SOLO no nosso Centro, entre 2009 e 2015. O follow-up clínico e ecocardiográfico foi aos seis meses de pós-operatório. A sobrevida e eventos major (deterioração valvular estrutural, disfunção valvular não estrutural, endocardite, reoperação, acidente vascular cerebral) foram aferidos através de registos clínicos e entrevista telefónica com o Cardiologista assistente até novembro de 2015 (follow-up médio 39±22 meses, máximo 78).
ResultadosA idade média dos doentes (n=345) foi 72±8 anos, 52% eram do sexo feminino e a mediana de euroSCORE II foi 2,7 (1,5–4,7). A mortalidade hospitalar foi 2,6%, não havendo mortalidade intra-operatória. O gradiente transvalvular médio pós-operatório e a média da área de orifício efetivo foram 11,9 ±4,5 mmHg e 1,9 ±0,5 cm2, respetivamente. O mismatch prótese-doente ocorreu em 14% dos casos, sendo um severo. A sobrevida cumulativa aos seis anos foi 72%. Seis indivíduos foram reoperados: três por endocardite infeciosa, dois por deterioração protésica e um por fístula periprotésica. Registaram-se cinco acidentes vasculares cerebrais, três endocardites tratadas farmacologicamente e um caso de deterioração valvular sem condições clínicas para reoperação. Não se registaram outras deteriorações valvulares estruturais ou disfunções valvulares não estruturais.
ConclusõesAs biopróteses stentless SOLO apresentaram resultados a curto e médio prazo promissores, revelando um excelente perfil hemodinâmico: baixos gradientes transvalvulares, áreas de orifício efetivo grandes e baixa incidência de mismatch prótese-doente.
atrial fibrillation
atrioventricular
aortic valve replacement
coronary artery bypass grafting
effective orifice area
Freedom Solo
mitral valve
patient-prosthesis mismatch
radiofrequency
Solo Smart
structural valve deterioration
tricuspid valve
Degenerative aortic valve disease is the most prevalent acquired heart valve disease in the western world. Surgical aortic valve replacement (AVR) is the therapy of choice for severe symptomatic disease and has become a safe procedure, reflecting not only advances in intra- and postoperative care, but also improvements in prosthetic valve design and technology.1,2 The ideal prosthetic valve should have low transvalvular gradients, maximum effective orifice area (EOA) and minimum patient-prosthesis mismatch (PPM), mimicking the anatomy and hemodynamic profile of healthy native valves. Moreover, it should be easy and safe to implant, durable, resistant to infection and have low thrombogenic risk. However, the search for the perfect artificial valve continues.3
Stentless bioprosthetic aortic valves, without an obstructive stent or a rigid suture ring, have proven excellent hemodynamic performance, similar to homografts. This improved hemodynamic profile is also associated with survival benefit, although this may be at the expense of a greater risk of structural valve deterioration (SVD) and need for reoperation.4 The first widely used stentless valve, the Toronto SPV, had low long-term durability, generally attributed to its stentless design, although it could also be related to its porcine origin.5 As with other early-generation stentless valves, implantation was technically demanding.6 The Freedom Solo (FS) and Solo Smart (SS) biological valves (Sorin Group, Saluggia, Italy) emerged in response to these technical challenges and have been in clinical use since 2004. These are third-generation stentless valves (the Smart is the same model with a different holder) with supra-annular implantation and a single suture line. The valves are manufactured from bovine pericardial tissue detoxified in homocysteic acid, which may reduce structural valve deterioration. As they are stentless, supra-annular and easily adaptable to the aortic root, they allow larger valve sizes and EOA than an equivalent stented valve, favoring laminar flow.7,8
High-volume centers have begun publishing their experience with these bioprostheses, confirming them as a safe and reliable alternative for AVR, but the available clinical data are limited and need to be supported by larger patient series and longer follow-up times.9–12
The objective of this study is to report the hemodynamic profile and the short- and medium-term outcomes of FS/SS stentless bioprosthetic valves implanted at our center during a six-year period.
MethodsStudy design and settingWe performed a retrospective, longitudinal and descriptive study. Clinical, demographic, operative and postoperative data were collected through medical records from the database of the Cardiothoracic Surgery Department of Centro Hospitalar São João. The study was approved by the local ethics committee.
Study populationPatients who underwent AVR with Freedom Solo or Solo Smart valves at Centro Hospitalar São João between April 2009 and April 2015 were identified from our center's registry, regardless of primary indication for surgery or concomitant procedures.
Preoperative data collected included age, gender, body surface area, body mass index, cardiovascular risk factors (hypertension, diabetes, dyslipidemia, smoking and obesity), creatinine clearance, peripheral arterial disease (defined as carotid occlusion or >50% stenosis, claudication, amputation or previous or planned intervention on the abdominal aorta, limb or carotid arteries), cerebrovascular events (transient ischemic attack or stroke), chronic obstructive pulmonary disease, coronary artery disease, left ventricular dysfunction, previous myocardial infarction, preoperative rhythm, New York Heart Association functional class and urgency of surgery. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was calculated for each patient. Information was also collected on both pathology (stenosis, regurgitation or combined) and etiology of aortic valve disease (degenerative calcific, bicuspid, endocarditis [native or prosthetic valve], rheumatic, prolapse or aortic prosthesis dysfunction).
The decision to use a stentless valve was at the discretion of the surgeons, after patient consent was obtained. Implantation of FS/SS valves was mainly performed by two senior surgeons (AFL-M and MJA), who considered all patients undergoing AVR with bioprosthetic valves for FS/SS implantation unless the following exclusion criteria were met: extensive aortic root calcification, severe mismatch between aortic annulus and sinotubular junction, or Sievers type 0 bicuspid aortic valve. Other types of bicuspid aortic valve were not excluded if aortic root symmetry was preserved.
Surgical and postoperative managementPatients underwent full or partial upper sternotomy and mild hypothermic cardiopulmonary bypass with cold crystalloid anterograde and retrograde cardioplegia. A transverse aortotomy was performed approximately 1 cm above the sinotubular junction. The aortic valve was excised and the annulus was completely decalcified and reinforced with a 5-0 polypropylene suture when necessary. Three 4-0 polypropylene sutures were placed in a supra-annular position at the nadir of each sinus and passed through the Solo valve. Thereafter, the valve was parachuted into the aortic root and tied with sutures running continuously 1 mm above the annulus. The sutures were passed out of the aorta at the level of the commissures and tied with the suture from the adjacent sinus. Immediate outcome was assessed by intraoperative transesophageal echocardiography. All patients underwent our center's standard anesthetic, surgical and postoperative care procedures. Surgical data were gathered regarding valve size and cardiopulmonary bypass and cross-clamp times for both isolated and combined procedures. Postoperative data collected are defined in the follow-up section.
Follow-upIn accordance with local protocol, patients had a postoperative six-month follow-up visit at our center that included transthoracic echocardiographic assessment. Mean gradients and EOA (calculated using the continuity equation) were recorded. PPM was classified by the ratio of prosthesis EOA to patient body surface area as moderate (0.85-0.65 cm2/m2) or severe (<0.65 cm2/m2).13 Thereafter, echocardiographic and clinical follow-up was carried out yearly by the patient's referring cardiologist.
In-hospital mortality (defined as 30-day mortality if the patient was discharged or within any period if the patient was not discharged14) was retrieved from hospital medical records. All-cause mortality (the primary outcome) was obtained from the National Healthcare Registry as of October 1, 2015. For the purposes of analysis, all cases of unknown cause of death were considered cardiovascular deaths.
Secondary endpoints were low cardiac output (need for high-dose inotropic support or intra-aortic balloon pump), stroke, acute renal function impairment (rise of serum creatinine >1.5 times the preoperative value or low urine output [<0.5 ml/kg/h for >6 hours]), atrial fibrillation episodes, permanent pacemaker implantation, severe thrombocytopenia (platelet count <30×109/L), early resternotomy for bleeding, prolonged ventilation (>24 hours mechanical ventilation), length of hospital stay, structural valve deterioration (SVD) or non-structural valve dysfunction, endocarditis or late reintervention for prosthesis-related issues. SVD was defined as changes intrinsic to the valve, such as wear, calcification, leaflet tear or suture line disruption of the valve's components, and non-structural valve dysfunction as any abnormality not intrinsic to the valve itself resulting in dysfunction of the operated valve or hemolysis.14 These data were also retrieved in October 2015, either from clinical records or telephone interview with the referring cardiologist. Due to lack of follow-up information, a post-hoc echocardiogram was performed at our center in 19 patients for the purpose of the current study.
Statistical analysisContinuous variables were expressed as mean (standard deviation) or median [interquartile range] (25th-75th percentile), as appropriate. Categorical variables were expressed as frequency and percentage. Comparisons between patients undergoing more than one procedure and those undergoing isolated AVR were performed using the unpaired Student's t test or the Mann-Whitney test for continuous variables. Normality was assessed by the Shapiro-Wilk test and visual inspection of residuals. Kaplan-Meier curves were used to assess time-to-event data. All statistical analyses were performed using IBM SPSS version 21 (IBM Corporation, New York). A p-value less than 0.05 was considered statistically significant.
ResultsSample and follow-upPreoperative characteristics of the study population are described in Table 1. Patients’ mean age was 72±8 years and 52% were female. The most prevalent cardiovascular risk factors were hypertension (79%) and dyslipidemia (68%); 35% of patients had diabetes, 25% were obese and 15% were current or former smokers. Degenerative calcific disease was the most common etiology for aortic valve disease (76%), 8% of patients had bicuspid and 8% rheumatic valves, and 5% had endocarditis (4% native valve, 1% prosthetic). The median EuroSCORE II was 2.7 [1.5-4.7]: 1.8 [1.1-3.1] for isolated AVR and 3.3 [2.2-6.4] for combined procedures. Out of 345 patients, 318 received FS and 27 SS. Medium-term clinical and echocardiographic follow-up (5±3 months) was retrieved from 98% of patients and was 100% (complete) for all-cause mortality. Mean follow-up was 39±22 months and maximum follow-up was 78 months.
Preoperative characteristics of the study population.
| Variables | n=345 |
|---|---|
| Age, years | 72±8 |
| Female gender | 178 (52) |
| Hypertension | 273 (79) |
| Diabetes | 121 (35) |
| Dyslipidemia | 235 (68) |
| Smoking | 52 (15) |
| BMI, kg/m2 | 27.7±4.5 |
| BSA, m2 | 1.8±0.2 |
| Obesity | 86 (25) |
| Renal impairment (CrCl <50 ml/min) | 97 (28) |
| PAD | 31 (9) |
| COPD | 66 (19) |
| Three-vessel CAD | 32 (9) |
| Moderate to severe LV dysfunction | 48 (14) |
| Previous MI | 45 (13) |
| Previous cerebrovascular event | 38 (11) |
| Preoperative rhythm | |
| Sinus rhythm | 257 (75) |
| AF | 77 (22) |
| Pacemaker | 11 (3) |
| NYHA class III-IV | 114 (33) |
| Aortic valve pathology | |
| Stenosis | 254 (74) |
| Regurgitation | 31 (9) |
| Combined | 60 (17) |
| Etiology | |
| Degenerative calcific | 263 (76) |
| Bicuspid | 27 (8) |
| Endocarditis (native valve) | 15 (4) |
| Endocarditis (prosthesis) | 5 (1) |
| Rheumatic | 27 (8) |
| Prolapse | 7 (2) |
| Aortic prosthesis dysfunction | 1 (0) |
| Urgent/emergent surgery | 64 (19) |
| EuroSCORE II | |
| Overall | 2.7 [1.5-4.7] |
| Isolated AVR | 1.8 [1.1-3.1] |
| Combined procedures | 3.3 [2.2-6.4] |
AF: atrial fibrillation; AVR: aortic valve replacement; BMI: body mass index; BSA: body surface area; CAD: coronary artery disease; CrCl: creatinine clearance; COPD: chronic obstructive pulmonary disease; LV: left ventricular; MI: myocardial infarction; NYHA: New York Heart Association; PAD: peripheral arterial disease.
The most frequently implanted valve size was no. 23 (39%), followed by sizes 25 (25%) and 21 (23%). One or more concomitant procedures were performed in 52% of patients (summarized in Table 2). Coronary artery bypass grafting (CABG) was the most frequent combined procedure (29%); mitral and tricuspid valves, as well as the ascending aorta, were also commonly treated. Compared with isolated AVR, combined procedures had longer median cardiopulmonary bypass and cross-clamp times, 95 [83-118] vs. 152 min [120-201] and 67 [59-85] vs. 110 min [85-140] (p<0.001), respectively. There was no intraoperative mortality.
Operative data.
| Variables | n=345 |
|---|---|
| Isolated AVR | 167 (48) |
| Combined procedures | |
| CABG | 101 (29) |
| Aorta surgery | 11 (3) |
| MV surgery | 54 (16) |
| TV surgery | 51 (15) |
| AF ablation by RF | 36 (10) |
| CPB time, min | |
| Isolated procedures | 95 [83-118] |
| Combined procedures | 152 [120-201] |
| Cross-clamp time, min | |
| Isolated procedures | 67 [59-85] |
| Combined procedures | 110 [85-140] |
AF: atrial fibrillation; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; MV: mitral valve; RF: radiofrequency; TV: tricuspid valve.
Overall in-hospital mortality was 2.6% (n=9), 1.8% (n=3) for isolated AVR and 3.4% (n=6) for combined procedures. Causes of death were prosthetic endocarditis (n=1), cardiogenic shock (n=2), septic shock (n=3), multiorgan failure (n=2) and iatrogenic complication of intensive care unit procedure (n=1). EuroSCORE II was lower in surviving patients (2.6 [1.5-4.6] vs. 10.0 [4.7-11.9], p=0.001).
Length of hospital stay was 7 [6-11] days and was significantly longer in patients who underwent combined procedures (8 [6-12] vs. 7 [6-9] days, p<0.001). Concerning in-hospital morbidity (Table 3), 30% of patients showed acute renal function impairment, 21% presented low cardiac output (requiring a high-dose single inotropic agent [9%], two or more inotropic agents [12%] or intra-aortic balloon pump [0.3%]), 7% had severe thrombocytopenia, 3% underwent pacemaker implantation due to atrioventricular (AV) conduction disturbances, and 2% suffered stroke. Only four patients (1%) underwent resternotomy for bleeding and none developed hemorrhagic stroke. In accordance with local protocol, patients without contraindication were discharged on vitamin K antagonists (changed to an antiplatelet agent three months after surgery); alternatively, patients were medicated with an antiplatelet agent.
Postoperative data.
| Variables | n=345 |
|---|---|
| In-hospital mortality | 9 (3) |
| Isolated AVR | 3 (2) |
| Combined procedures | 6 (3) |
| Hospital length of stay (days) | 7 (6-11) |
| Prolonged ventilation (>24 hours) | 16 (5) |
| Low cardiac output | 73 (21) |
| Stroke | 6 (2) |
| Severe thrombocytopenia | 25 (7) |
| Resternotomy for bleeding | 4 (1) |
| Acute renal impairment | 104 (30) |
| New-onset AF | 113 (44) |
| Permanent pacemaker implantation | 9 (3) |
AF: atrial fibrillation; AVR: aortic valve replacement.
Mean transvalvular gradient was 11.9±4.5 mmHg and EOA was 1.9±0.5 cm2 (Figure 1). PPM occurred in 38 patients (13.7%) and was severe in only one case. This patient had a BSA of 1.86 m2, a number 23 valve was implanted and the mean transprosthetic gradient on follow-up echocardiographic assessment was 13 mmHg. Mean transprosthetic gradient in patients with moderate or severe PPM was 16.3±5.6 mmHg, EOA was 1.33±0.18, BSA was 1.78±0.17 m2 and the most frequent prosthesis size was 21. Three cases of SVD were identified during follow-up (two of these patients were reoperated).
Forty-seven patients (14%) died after discharge. The underlying cause was non-cardiovascular in 25 patients and cardiovascular in 22. Two deaths related to SVD were identified: one patient died after reoperation and the other was considered unsuitable for reintervention. The one-, three-, and six-year cumulative survival rate was 94%, 87% and 72%, respectively. Patients who underwent isolated AVR showed better survival than those who underwent combined procedures (p=0.005, log-rank test) (Figure 2).
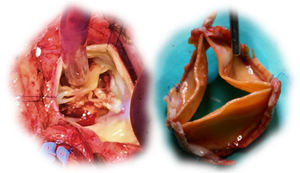
Six patients were reoperated: three due to endocarditis (two, 15 and 19 months after surgery), two for SVD (41 and 67 months after surgery) and one for periprosthetic fistula (two months after surgery). Two cases of prosthetic endocarditis were attributed to Staphylococcus aureus and one to Enterococcus faecalis infections; extensive root and subvalvular abscesses were found, with small or no vegetations on the cusps (two of these patients required root replacement due to extensive tissue infiltration). In the two cases of SVD (a 59-year-old male and a 67-year-old female), the surgeon found immobile and severely calcified cusps, but with only mild to moderate thickening. A visible delamination plane between the prosthesis pericardium and the native aortic root enabled easy en bloc explantation of the valves (Figure 3). The periprosthetic fistula was due to fracture of the 4-0 polypropylene suture in the right coronary sinus, but there was no valve deterioration or root dilation, and so the leak was closed with a continuous 4-0 polypropylene suture.
Throughout follow-up, five strokes and three cases of endocarditis, treated medically, were recorded. Loss of follow-up occurred in eight cases (2.8%) (Table 4).
DiscussionOur center's experience in a series of 345 consecutive patients who underwent AVR with FS/SS bioprosthetic valves is similar to previously published results.9–12,15,16
Except for patients with extensive aortic root calcification, severe mismatch between the aortic annulus and sinotubular junction and Sievers type 0 bicuspid aortic valve, all patients were considered for FS/SS implantation by our center's two senior surgeons, notwithstanding primary indication for surgery or concomitant procedures. This indicates that these stentless valves are widely applicable. Specifically, other types of bicuspid valve disease (8% of patients) and endocarditis (5%) were not exclusion criteria for FS/SS implantation provided that aortic root symmetry was preserved.
Our sample included a higher percentage of females, similar to other series using stentless valves, as was reported in a recent systematic review and meta-analysis on surgical AVR.4 According to this review, in studies on stented bioprostheses, the proportion of males is higher (61.9% vs. 55.0% for stentless bioprostheses). Also noteworthy is the different proportion of concomitant CABG procedures (41.5% vs. 28.9% in studies of stented vs. stentless bioprostheses, respectively). Moreover, it reported that early and late mortality are lower in studies on stentless valves, in accordance with the hypothesis that their hemodynamic superiority results in survival benefits compared with stented bioprostheses, but this may reflect a patient selection bias.4
The implantation technique proved to be simple and fast, with a similar mean cross-clamp time for isolated AVR (67 [59-85] min) to previous studies on the Solo valve.17 These times are shorter than those observed with earlier generations of stentless aortic valves (72-128 min) and also comparable to those reported for stented valves (50-67 min).17–20
In-hospital morbidity and mortality rates were low and comparable to previous studies, confirming the safety of FS/SS.7–10 Thrombocytopenia has been reported to be associated with FS/SS valves and may cause concern.21 The precise mechanism remains to be identified, although Stanger et al. suggest that a temporary chemistry-induced lysis underlies this phenomenon.22 We observed a mean 65% decrease in platelet count after implantation and 7% of patients showed severe thrombocytopenia. Despite this transient thrombocytopenia, only four patients underwent early resternotomy for bleeding and no episodes of hemorrhagic stroke were observed.
The FS/SS supra-annular implantation technique is believed to reduce the incidence of postoperative permanent pacemaker implantation due to AV conduction disturbances, with previously published numbers between 1.3% and 2.7%, lower than those reported for stented prostheses (7% for isolated AVR).9–12 In our series, nine individuals (2.7%) underwent definitive pacemaker implantation; of these, six underwent combined procedures and one had active endocarditis.
Recent reports on FS/SS valves have shown auspicious hemodynamic outcomes.15,16,19 Our assessment of these valves’ hemodynamic performance was carried out by transthoracic echocardiography 5±3 months after surgery. The mean transvalvular gradient was 11.9±4.5 mmHg and mean EOA was 1.9±0.5 cm2. These findings were consistent with those reported in previous publications on the hemodynamic profile of the FS/SS (mean pressure gradient 7.2±4.0 mmHg at one year, mean EOA 1.5±0.5 cm2 at one year).11 Other studies have described similar or higher mean gradients in stented aortic bioprosthetic valves (10-16 mmHg).20 Moreover, according to a 2016 study, the Solo stentless valve provides better short- and medium-term hemodynamic performance than the stented Carpentier-Edwards bioprosthetic valve.16 The overall rate of PPM in our series was low (13.7% of patients), severe in only one case, which clearly demonstrates the excellent hemodynamic profile of the valve, as previously reported (overall PPM 9.8%; severe PPM 1.3%).9
In our series, freedom from reoperation at six years reached 95.9%, similar to the medium-term results of Wollersheim et al. with Solo valves (96% freedom from aortic valve reoperation at six years).9 On the other hand, Stanger et al. reported a higher reoperation rate, with explantation of 14 of 149 Solo valves, representing 72% freedom from aortic valve reoperation at nine years. Freedom from SVD in our series was 97.1%, slightly lower than the 98% found by Wollersheim et al. in their 350-patient series.9 Stanger et al. reported 26 cases of SVD out of 149 patients (17%), 10 of them requiring reoperation, representing less than 75% freedom from SVD at nine years of follow-up.22 Although medium-term outcomes seem promising, a six-year period is insufficient to draw conclusions regarding long-term durability. The durability of this bioprosthetic valve needs to be studied more thoroughly, with longer follow-up and larger samples. However, a recent multicenter study with a 10-year follow-up provides evidence of the long-term durability and hemodynamic performance of the FS valve.15
Our cumulative survival rate (72% at six years) was similar to literature reports (74-80% at five years) for FS and other aortic valves.17,23,24 As expected, the survival of patients with isolated AVR was significantly better than that of patients undergoing combined procedures.
Study limitationsThis work has the limitations inherent to any retrospective study. Additionally, it was a single-center study prone to selection bias because the choice of prosthesis was based on the surgeons’ preference. Finally, longer follow-up times are warranted to assess long-term durability.
ConclusionsTo the best of our knowledge, this is one of the largest single-center series with FS and SS stentless aortic valves. These results support previous publications, showing that these prostheses are safe to implant, with good short- and medium-term clinical outcomes. Moreover, they should be considered a reliable alternative for AVR, as they demonstrate an excellent hemodynamic performance: low transvalvular gradients, large EOA and low incidence of PPM.
Funding statementThis article is a result of the project DOCnet (NORTE-01-0145-FEDER-000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). F. Saraiva was supported by Universidade do Porto/FMUP and FSE-Fundo Social Europeu, NORTE 2020-Programa Operacional Regional do Norte,NORTE-08-5369-FSE-000024-Programas Doutorais.
Conflicts of interestThe authors have no conflicts of interest to declare.










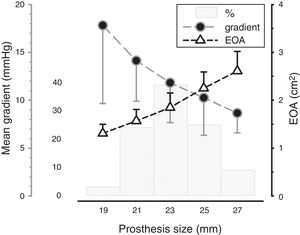 EOA: effective orifice area.'/>
EOA: effective orifice area.'/>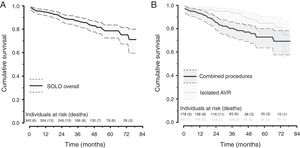 AVR: aortic valve replacement.'/>
AVR: aortic valve replacement.'/>