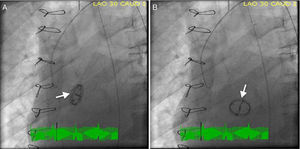
We report the case of a 62-year-old man with a previous mechanical aortic valve prosthesis who was referred for emergent cardiac catheterization after successful resuscitation from an out-of-hospital pulseless electrical activity cardiac arrest. Post-resuscitation, the patient was comatose with arterial blood pressure 60/35 mmHg, and the electrocardiogram showed widespread ST-segment depression and ST-elevation elevation in lead aVR. Mechanical ventilation, inotropic and vasopressor support were initiated during the transfer to a percutaneous coronary intervention-capable hospital. Cardiac fluoroscopy showed pivotal movement of the aortic prosthesis with each heartbeat and severe regurgitation was seen on aortography (Video 1). Prosthesis position in systole (Figure 1A) and diastole (Figure 1B) was demonstrated in individual cinefluoroscopic frames (left anterior oblique view). In diastole, the blood regurgitated into the left ventricular cavity produced a 90° clockwise rotation of the aortic prosthesis. Coronary angiography was normal. The patient underwent emergent cardiac surgery, during which the intraoperative transesophageal echocardiogram showed a large nodular structure suggestive of vegetation at the aortic prosthesis leaflet in addition to severe aortic regurgitation (Video 2). Macroscopically, the prosthesis was found to be three-fourths detached and there were ring and perivalvular tissue vegetations with annular abscesses. Excision and replacement of the dehisced aortic valve was performed. Methicillin-resistant Staphylococcus epidermidis was grown from peripheral blood and prosthetic material cultures. After completing four weeks of vancomycin therapy, the patient was discharged home asymptomatic.
Prosthetic aortic valve detachment is a rare complication, occurring in 0.1%–1.3% of patients who undergo aortic valve replacement.1 The known risk factors for detachment are bacterial endocarditis, concomitant aneurysm of the ascending aorta, and severe calcification of the native aortic valve. Although echocardiography is the modality of choice for evaluating cardiac valves, assessment of mechanical prosthetic valve function by echocardiography is sometimes difficult. The value of gradients in isolation is limited, since gradients depend not only on flow magnitude but also on valve type and size.2 High transprosthetic velocity alone is not proof of intrinsic aortic valve prosthesis obstruction, but may be secondary to high flow, prosthesis-patient mismatch, or pressure recovery at the smaller central valvular orifice. Conversely, high transprosthetic gradients may not be evident in cases of low cardiac output, even in the presence of obstruction.3 In cases of suspected dysfunctional prosthesis, visualization of prosthetic leaflet motion can help in differential diagnosis. However, the ability of echocardiography to visualize prosthesis disc motion is limited by the acoustic shadowing caused by the prosthetic valve itself.
Our case demonstrates that fluoroscopy permits rapid and easy evaluation of mechanical prosthetic valve function, enabling normal and dysfunctional prostheses to be distinguished. It has been demonstrated that fluoroscopy, particularly in aortic position, is superior to two-dimensional echocardiography in identifying disc motion, and so for this purpose, cinefluoroscopy is considered the gold standard technique.3 In conclusion, cinefluoroscopy rapidly provides valuable information to assess prosthetic valve function which is complementary to that obtained by echocardiography.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.