Fungal prosthetic valve endocarditis is an extremely severe form of infective endocarditis, with poor prognosis and high mortality despite treatment. Candida albicans is the most common etiological agent for this rare but increasingly frequent condition.
We present a case of fungal prosthetic valve endocarditis due to C. albicans following aortic and pulmonary valve replacement in a 38-year-old woman with a history of surgically corrected tetralogy of Fallot, prior infective endocarditis and acute renal failure with need for catheter-based hemodialysis. Antifungal therapy with liposomal amphotericin B was initiated prior to cardiac surgery, in which the bioprostheses were replaced by homografts, providing greater resistance to recurrent infection. During hospitalization, a mycotic aneurysm was diagnosed following an episode of acute arterial ischemia, requiring two vascular surgical interventions. Despite the complications, the patient's outcome was good and she was discharged on suppressive antifungal therapy with oral fluconazole for at least a year.
The reported case illustrates multiple risk factors for fungal endocarditis, as well as complications and predictors of poor prognosis, demonstrating its complexity.
A endocardite fúngica de prótese valvular é uma forma extremamente severa de endocardite infeciosa, com mau prognóstico e elevada mortalidade apesar do tratamento médico. Candida albicans é o agente etiológico mais frequentemente implicado nesta rara, embora crescente, patologia.
Relata-se um caso de endocardite fúngica de prótese valvular após substituição das válvulas aórtica e pulmonar numa mulher de 38 anos com antecedentes de tetralogia de Fallot cirurgicamente corrigida, endocardite infeciosa prévia e insuficiência renal aguda com necessidade de hemodiálise por catéter. A terapêutica antifúngica com anfotericina B lipossómica foi iniciada previamente à cirurgia cardíaca, onde as biopróteses foram substituídas por homoenxertos, conferindo maior resistência à infeção recorrente. Durante o internamento, foi diagnosticado um aneurisma micótico, na sequência de um episódio de isquemia arterial aguda, obrigando a duas intervenções pela Cirurgia Vascular. Apesar das complicações, a doente teve um bom outcome clínico e recebeu alta com terapêutica de erradicação com fluconazol durante, pelo menos, um ano.
O caso relatado enumera múltiplos fatores de risco para endocardite fúngica, assim como complicações e preditores de mau prognóstico, ilustrando a sua complexidade.
Fungal endocarditis (FE) is a rare condition with high mortality and poor prognosis.1Candida albicans is the most frequent causative agent.2 Among the risk factors for fungemia and FE are prosthetic heart valves, prolonged use of central venous catheters, parenteral nutrition, exposure to broad-spectrum antibiotics and immunosuppression.3Candida endocarditis is more commonly found in patients with prosthetic heart valves and occurs more often in patients with a history of prior endocarditis4 and early in the postoperative period.5 Treatment includes prompt institution of antifungal therapy, valve replacement and long-term eradication therapy.
Case reportA 38-year-old woman with a history of surgically corrected tetralogy of Fallot at the age of 11, and recent dental procedures, presented with fever, anorexia, weight loss and fatigue for one month and epigastric pain of recent onset.
On physical examination the patient was pale and presented with bilateral lower limb edema, jugular distension and a grade II/IV diastolic murmur on the left sternal border. Laboratory tests showed elevated C-reactive protein (CRP), anemia and thrombocytopenia. Blood cultures were negative. Abdominal computed tomography (CT) revealed hepatomegaly and splenomegaly with splenic infarct, thought to result from septic embolism. The transthoracic echocardiogram (TTE) revealed an extremely mobile 15-mm vegetation on the right coronary cusp of the aortic valve and severe aortic regurgitation, accompanying previously known severe dilation of the right heart chambers and pulmonary regurgitation.
A diagnosis of infective endocarditis was made and empirical antibiotic therapy was initiated with amoxicillin-clavulanate and gentamicin, prior to transfer to our hospital. After 17 days of antibiotic therapy, signs of congestive heart failure and infection persisted and the patient was referred to our cardiothoracic surgery center. Replacement of the aortic and pulmonary valves with 23- and 25-mm Carpentier-Edwards Perimount Magna® bioprostheses, respectively, reconstruction of the right ventricular outflow tract, and tricuspid valve ring annuloplasty were successfully performed.
In the postoperative period, the patient presented with nonoliguric acute renal failure and continuous renal replacement therapy (catheter-based hemodialysis) was instituted. The patient was discharged 53 days after admission on a hemodialysis program and with no evidence of prosthetic infection on TTE.
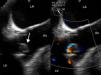
Three days after discharge she was readmitted with fever and dyspnea, along with elevated CRP and anemia. Blood cultures were positive for C. albicans. Antifungal therapy with liposomal amphotericin B was initiated but subsequent blood cultures remained positive. A transesophageal echocardiogram (TEE) demonstrated large vegetations on the aortic valve and a periprosthetic leak resulting in mild aortic regurgitation, establishing the diagnosis of FE (Figure 1).
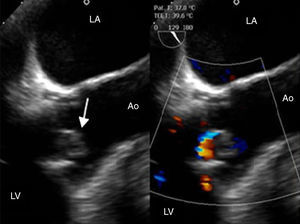
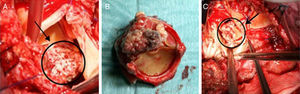
At redo surgery the pulmonary prosthesis was also found to contain large vegetations (Figure 2) and both prosthetic valves were replaced by cryopreserved homografts. Microbiological analysis of the removed tissue revealed C. albicans.
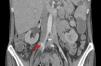
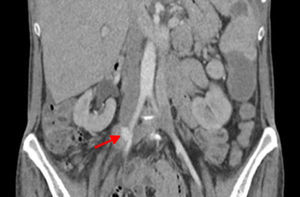
In the postoperative period blood cultures became negative and renal function recovered. Due to pancytopenia, liposomal amphotericin B therapy was switched to intravenous fluconazole. Five days after surgery, the patient suffered acute ischemia of the right lower limb and right iliofemoral embolectomy was performed by vascular surgery. Direct examination of the thrombus revealed C. albicans hyphae. TTE and cardiac magnetic resonance imaging showed no signs of cardiac infection. Thoracoabdominal CT revealed a pseudoaneurysm of the right iliac artery (Figure 3), the probable source of the emboli, and the patient was reoperated, with resection of the aneurysm and replacement by a segment of reversed saphenous vein. Clinical pathological analysis confirmed its infectious etiology and the diagnosis of mycotic aneurysm.
The patient's condition improved and she was discharged eight weeks after the second cardiac surgery, on suppressive antifungal therapy with oral fluconazole and surveillance by imaging studies and blood cultures. Expected time of treatment will be at least a year.
DiscussionProsthetic valve endocarditis (PVE) is the most severe form of infective endocarditis, accounting for 10-30% of all cases.1 Health care-associated infections have been found to account for 37% of PVE.6 The risk is higher in the first year after valve replacement surgery for both mechanical and bioprosthetic valves.
Fungal etiology accounts for 2-4% of all cases of endocarditis.7 Its incidence has increased in recent decades due to a growing number of patients at risk.8 It is associated with worse prognosis1 and 50% mortality rate despite treatment.2,3
PVE is a diagnostic challenge: the modified Duke criteria9 lack sensitivity in this context, blood cultures are more often negative and echocardiography has less diagnostic accuracy.1 Therefore, a high index of suspicion is needed for its diagnosis, in order to enable early initiation of therapeutic measures that can improve the prognosis.
In the case reported, no vegetations were identified in the pulmonary prosthesis by echocardiography. This is presumably due to the poor visualization of the pulmonary prosthesis by TEE and to its lower sensitivity in cases of PVE.
TEE is more sensitive and useful in cases of PVE.10 FE usually leads to larger vegetations than those found in bacterial endocarditis.10 As a result, arterial embolization, as presented by our patient, is more common,11 the usual sites being the cerebral circulation, extremities and gastrointestinal tract.
No randomized studies have assessed the optimal therapy for fungal PVE. The therapeutic approach is based on antifungal agents and valve replacement surgery, although successful treatments of FE have been reported with medical therapy alone.12 Moreover, no uniform recommendations are available concerning the most appropriate antimicrobial regimen, the ideal timing for surgery or the total duration of treatment. According to the ESC guidelines, surgery may be undertaken on an elective or urgent basis depending on the patient's condition,1 although others recommend earlier surgery, as it appears to improve outcome.13 In the case presented, an initial approach with antifungal therapy was adopted, due to the high risk associated with a third cardiac surgery (EuroSCORE II of 38.5), but the persistent fungemia made surgery advisable.
In our patient, amphotericin B was used in a lipid formulation to avoid further deterioration of renal function, but the development of pancytopenia forced conversion to fluconazole. Since the potential for recurrent infection is extremely high in fungal PVE, suppressive therapy with fluconazole is recommended for prolonged periods or even indefinitely.10
Given the high recurrence rate of FE, the use of homografts is justified by their higher resistance to recurrent infection. This advantage may be explained by the suggestion that homografts have the ability to reproduce collagen, improving postoperative local healing.14 Homografts allow complete debridement of all infected tissue and better tissue penetration of antimicrobial agents, facilitating eradication of the infection.14
The source of our patient's candidemia is unclear, but the long hospitalization, the presence of a recent implanted prosthetic valve, and an indwelling central venous catheter for the hemodialysis program represent risk factors that may explain it. The patient's congenital heart disease is also a predisposing factor for infective endocarditis.
In this case numerous predictors of poor outcome were present, besides the fungal etiology of a prosthetic valve infection. These included the clinical manifestations of congestive heart failure, the long period of hospitalization with central venous catheters, renal failure requiring hemodialysis, the large size of the fungal vegetations, carrying higher embolic risk, and finally the mycotic pseudoaneurysm of the right iliac artery.
ConclusionFE caused by C. albicans is a rare condition with poor prognosis. The presence of a prosthetic valve and a central venous catheter are major risk factors for FE. The diagnosis is challenging as only about half of blood cultures are positive and the sensitivity of echocardiography in prosthetic valve endocarditis is limited. Despite the lack of evidence from randomized clinical trials, treatment combining antifungal therapy and valve replacement is thought to offer improved clinical outcomes. The timing of surgery is not consensual but early surgery appears to improve survival, depending on the patient's clinical condition. Moreover, given the high recurrence rate of fungal PVE, homografts appear to be the most appropriate choice, providing the highest resistance to recurrent infection, by allowing complete debridement of infected tissue with low risk of valve dehiscence and better antibiotic penetration.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.