Myocarditis is defined as inflammation of the myocardium. The clinical manifestations of myocarditis vary from flu-like symptoms to fatal fulminant forms.
We report the case of a 39-year-old woman with a diagnosis of cardiogenic shock caused by fulminant myocarditis. Extracorporeal membrane oxygenation was used as a bridge to recovery.
Etiological study revealed Legionella pneumophila serogroup 1 infection.
Recovery of biventricular function was seen after treatment with azithromycin.
A miocardite é uma doença inflamatória do miocárdio. Pode-se apresentar de forma subtil ou fulminante.
Apresenta-se o caso duma mulher de 39 anos com choque cardiogénico no contexto de miocardite fulminante, com necessidade de colocação de membrana de oxigenação extracorporal artério-venosa como ponte para a recuperação. O estudo etiológico revelou infeção por legionella pneumophila do serogrupo 1. Após início de antibioterapia com azitromicina endovenosa verificou-se recuperação da função biventricular.
Myocarditis is defined as inflammation of the myocardium. Its cause may be infectious (viral, bacterial, protozoal, fungal) or non-infectious (autoimmunity, drugs, toxins, hypothermia, or radiation). Enterovirus is the most commonly isolated agent but the etiology is generally not identified.1 Myocarditis is often asymptomatic or may be accompanied by non-specific symptoms such as fever, myalgia, palpitations, chest pain or dyspnea, but it may be manifested by heart failure, cardiogenic shock (CS), cardiopulmonary arrest or sudden death. It is the cause of 8.6–12% of sudden deaths in young adults.2,3
Diagnosis is based on clinical criteria and non-invasive diagnostic exams; endomyocardial biopsy (EMB) is reserved for specific situations.1
The non-specific and varied nature of presentation means that the real incidence is difficult to determine; it has been estimated at 0.12–12%1 in autopsy reports.
There are no known predictors of its course, but certain clinical markers are associated with worse outcome: NYHA class III or IV, bundle branch block, pulmonary hypertension, syncope, low mean arterial pressure, and left ventricular ejection fraction <40%.3 Hemodynamic support is recommended with ventricular assist devices or extracorporeal membrane oxygenation (ECMO) as a bridge to transplantation or recovery in cases of refractory shock.1,3
Case reportA 39-year-old Caucasian woman, allergic to aspirin and not taking regular medication, was admitted to a cardiothoracic intensive care unit (CTICU) for ECMO with a diagnosis of cardiogenic shock (CS) in the context of fulminant myocarditis. She had been sedated with midazolam and was under mechanical ventilatory support.
The patient had had CS for 48 hours, with renal failure (continuous venovenous hemofiltration had been started the day before) and liver failure refractory to treatment with amines (noradrenaline and dobutamine) and intra-aortic balloon pump (IABP).
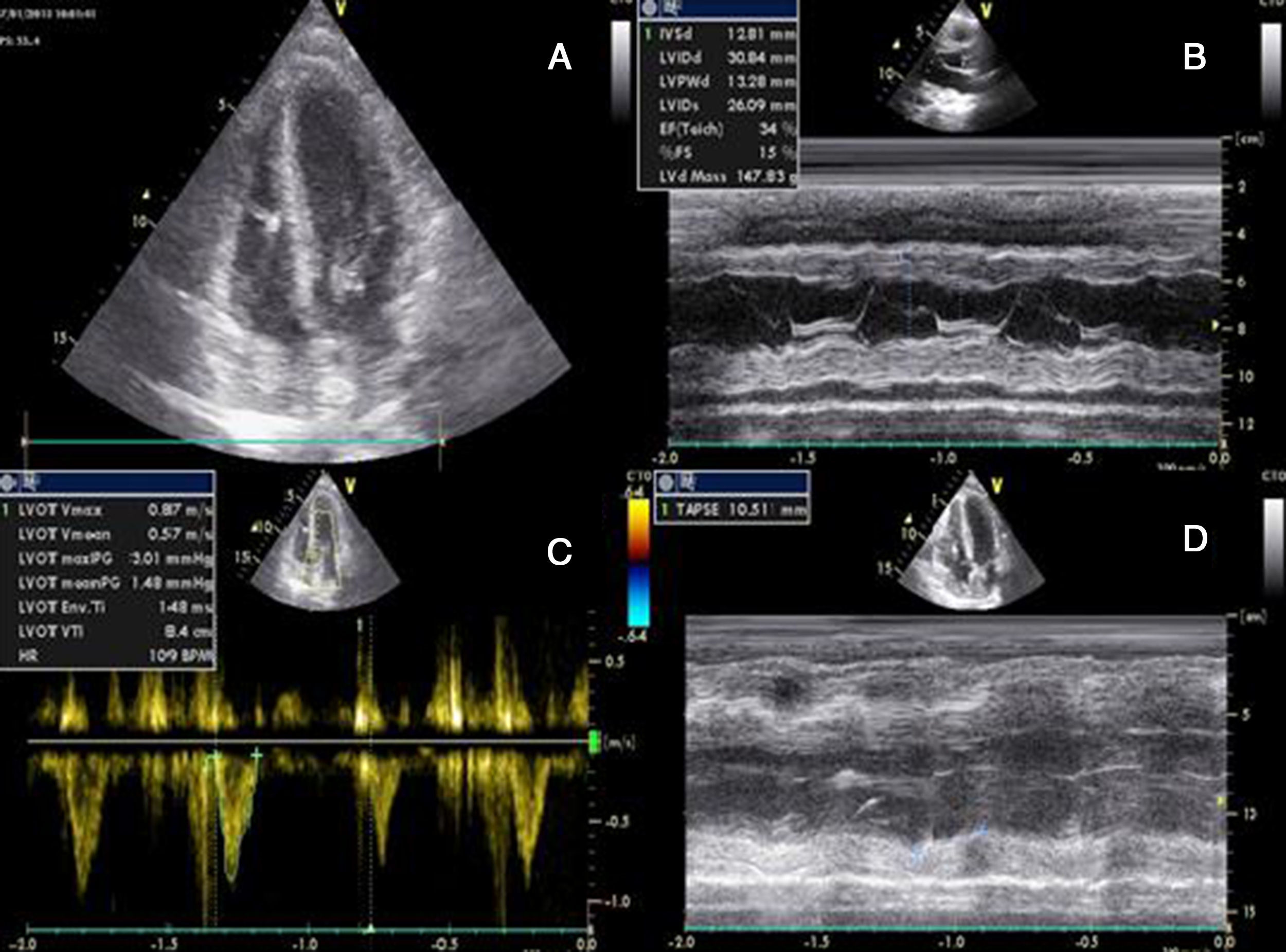
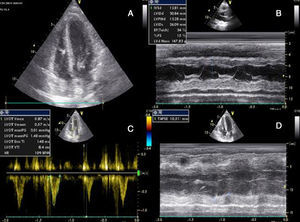
She had gone to the emergency department (ED) 48 hours before due to syncope with spontaneous recovery and flu-like symptoms for seven days. There were no alterations on initial physical examination, electrocardiogram (ECG) or brain computed tomography (CT). Laboratory tests showed increased inflammatory parameters (white cell count 23900/m3 with 85% neutrophils and C-reactive protein 2.83 mg/dl) and elevated troponin I (2 μg/l). During observation in the ED the patient developed shock and was transferred to the general intensive care unit, where transthoracic echocardiography (TTE) revealed non-dilated cardiac chambers, mild hypertrophy of the left ventricular (LV) walls, biventricular dysfunction and mild circumferential pericardial effusion (Figure 1).
Transthoracic echocardiography after onset of shock, showing biventricular dysfunction and mild circumferential pericardial effusion. (A) Apical 4-chamber view showing mild hypertrophy of the left ventricular walls (probably edema); (B) M-mode assessing ventricular dimensions and left ventricular function; (C) left ventricular outflow tract velocity-time integral, an indirect measure of left ventricular function; (D) right ventricular function assessed by tricuspid annular plane systolic excursion. LVOT: left ventricular outflow tract; TAPSE: tricuspid annular plane systolic excursion; VTI: velocity-time integral.
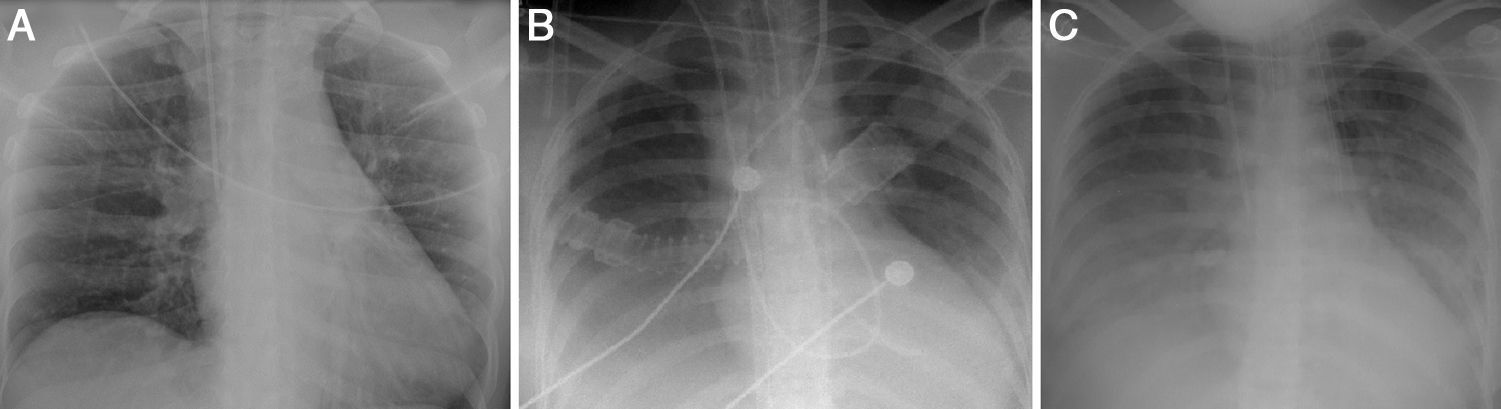
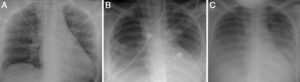
A diagnosis of CS was made, confirmed by Swan-Ganz catheter, which showed cardiac output of 2 l/min, cardiac index of 1.2 l/min/m2, and systemic vascular resistance of 1438 dyn s/cm5. A provisional diagnosis of fulminant myocarditis was made; the pericardial effusion raised the suspicion that the pericardium was involved, but there was no ECG evidence or history of pain that would suggest pericarditis. The chest X-ray at admission showed no abnormalities (Figure 2A).
On admission to the CTICU the patient showed signs of poor peripheral perfusion, necrotic lesions on the big toes, and bilateral ankle edema. She was tachycardic, in sinus rhythm, at 120 bpm and hypotensive (mean arterial pressure [MAP] 50 mmHg), under inotropic support with dobutamine 15 μg/kg/min and noradrenaline 20 μg/kg/min, and with IABP 1:1.
TTE was repeated and confirmed the echocardiographic findings.
Veno-arterial ECMO was established (with debridement of the right femoral vein and artery) and cardiac output was programmed for 5 l/min. MAP rose to 60 mmHg but noradrenaline was discontinued due to poor peripheral perfusion, attributed to shock and high vasopressor dosages, and dopamine was begun 24 hours after admission at a maximum dose of 5 μg/kg/min.
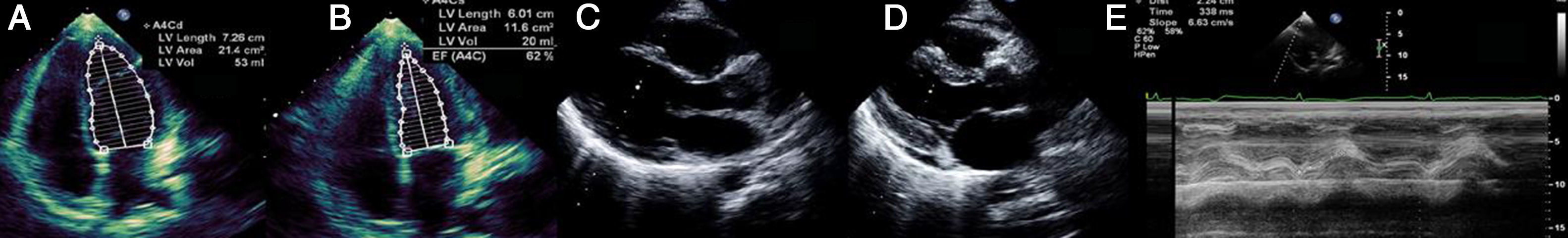
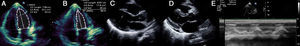
Laboratory tests requested at admission as part of the pre-transplantation protocol (transplantation being the next step in the event of persistent shock) included etiological study of myocarditis and 48 hours after admission urine antigen tests were positive for Legionella pneumophila serogroup 1. Treatment was begun with intravenous azithromycin. The X-ray showed right pleural effusion (Figure 2); 72 hours after beginning antibiotic therapy the patient presented improved hemodynamic stability and TTE was performed (under inotropic support with dobutamine 13 μg/kg/min, dopamine 3 μg/kg/min, IABP at 2:1 and ECMO) that showed recovery of biventricular function (Figure 3). The IABP and ECMO were withdrawn, the patient remaining hemodynamically stable.
Transthoracic echocardiography showing recovery of biventricular function. (A) and (B) Left ventricular function assessed by Simpson's biplane method; parasternal long-axis view in systole (C) and diastole (D), showing reduction in left ventricular wall hypertrophy and pericardial effusion; (E) right ventricular function assessed by tricuspid annular plane systolic excursion.
On the seventh day after admission the patient suffered acute lower limb ischemia, more severe on the left, secondary to cannulation of the femoral arteries, use of high-dose vasopressors and low cardiac output. External and internal fasciotomy of the left limb and external fasciotomy of the right were performed 48 hours later.
On day 10, despite inotropic support, the patient began to suffer periods of hypotension and a rise in inflammatory parameters. TEE under the same inotropic support as before showed no alterations, but hemodynamic assessment by Swan-Ganz catheter suggested distributive shock, with cardiac output 7 l/min, central venous pressure 13 mmHg, cardiac index 4.16 l/min, pulmonary capillary wedge pressure 15 mmHg, mean pulmonary artery pressure 22 mmHg, and systemic vascular resistance 466 dyn s/cm5.
A provisional diagnosis of sepsis of unknown origin was made and empirical antibiotic therapy was begun with meropenem after collection of blood and urine samples. The fasciotomies were assumed to be the site of infection.
As MAP was consistently lower than 60 mmHg, noradrenaline was administered for around 24 hours at a maximum dose of 10 μg/min and dobutamine was discontinued. Optimization of the antibiotic regime and recovery of biventricular function resulted in hemodynamic stabilization and inotropic support and sedation were withdrawn; the patient was successfully extubated on the 10th day of hospitalization.
No ECG alterations were detected; the patient still required venovenous hemofiltration due to oligoanuria.
On day 14 she was transferred back to the original hospital for plastic and vascular surgery and nephrological treatment.
TTE at discharge, without inotropic support, confirmed recovery of biventricular function. The etiological study of myocarditis showed no other alterations, and so a diagnosis was made of fulminant myocarditis caused by L. pneumophila serogroup 1. Although there was no evidence on the X-ray (Figure 2) of condensation, the persistence of pleural effusion despite improvement in ventricular function suggests an infectious component in addition to the blood pooling due to heart failure (although this could have been aggravated by continuing renal failure), and the effusion could have masked any condensation. However, there was no evidence of bacterial pneumonia (Table 1).
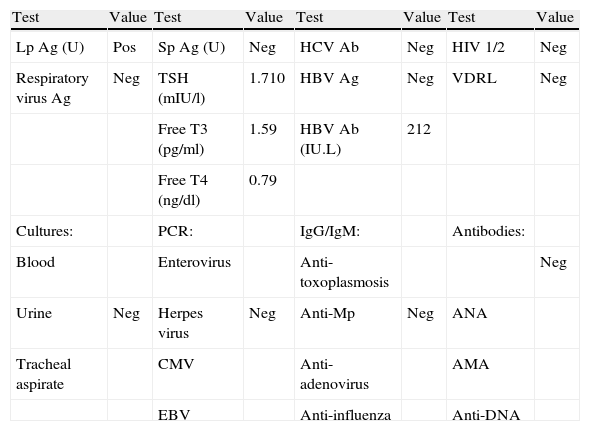
Laboratory tests performed as part of the pre-transplantation protocol.
| Test | Value | Test | Value | Test | Value | Test | Value |
| Lp Ag (U) | Pos | Sp Ag (U) | Neg | HCV Ab | Neg | HIV 1/2 | Neg |
| Respiratory virus Ag | Neg | TSH (mIU/l) | 1.710 | HBV Ag | Neg | VDRL | Neg |
| Free T3 (pg/ml) | 1.59 | HBV Ab (IU.L) | 212 | ||||
| Free T4 (ng/dl) | 0.79 | ||||||
| Cultures: | PCR: | IgG/IgM: | Antibodies: | ||||
| Blood | Enterovirus | Anti-toxoplasmosis | Neg | ||||
| Urine | Neg | Herpes virus | Neg | Anti-Mp | Neg | ANA | |
| Tracheal aspirate | CMV | Anti-adenovirus | AMA | ||||
| EBV | Anti-influenza | Anti-DNA |
Ab: antibodies; Ag: antigen; AMA: antimitochondrial antibodies; ANA: antinuclear antibodies; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; Lp: Legionella pneumophila; Mp: Mycoplasma pneumoniae; Neg: negative; PCR: polymerase chain reaction; Pos: positive; Sp: Streptococcus pneumoniae; TSH: thyroid-stimulating hormone; U: urine; VDRL: Venereal Disease Research Laboratory diagnostic test for syphilis.
The absence of problems with ventilation or oxygenation and of purulent secretions in the tracheobronchial tree is evidence against the existence of a pulmonary focus of infection.
Thoracic CT was not performed due to the patient's initial hemodynamic instability and subsequent fasciotomies of the lower limbs.
The pericardial effusion may have had the same etiology as the pleural effusion – infection, pericarditis (although as pointed out above there was no history of chest pain or ECG evidence), or fluid overload due to heart and renal failure.
DiscussionMost cases of myocarditis have a benign course, but some patients progress rapidly to CS, as in the case described.
The Dallas criteria for the diagnosis of myocarditis, published in 1986, were based on the type, extent and distribution of inflammatory infiltrate and myocyte necrosis in EMB samples.1 In 1991, Lieberman et al. presented a clinicopathologic classification of myocarditis: fulminant, subacute, chronic active and chronic persistent.4
Fulminant myocarditis presents with a flu-like prodrome followed by severe and sudden cardiovascular compromise associated with ventricular dysfunction.4
Although histologic findings are part of the Dallas criteria and in those proposed by Lieberman, EMB is reserved for specific situations. According to the AHA/ACCF/ESC guidelines,5 it could have been considered in the case presented if shock had persisted, in order to identify causes that require specific therapy and that can only be diagnosed by histologic study, such as giant cell myocarditis and infiltrative cardiomyopathy.
In the present case, the diagnosis was based on clinical criteria. TTE was important to identify the type of shock, exclude other causes of CS (tamponade, valvular disease, tumor, Takotsubo or restrictive cardiomyopathy, endocarditis or LV outflow tract obstruction), and to monitor the response to therapy.
Cardiac magnetic resonance imaging is the exam of choice in suspected myocarditis,2 but cannot be performed at the patient's bedside.
The echocardiographic findings were in line with those reported in other cases. In a study by Pinamonti et al.6 in patients with fulminant myocarditis, LV dysfunction was seen in 69% of cases and right ventricular dysfunction in 23%; LV dilatation was rare. LV hypertrophy was seen in four of these patients, also observed by Felker et al.,7 associated with the marked inflammatory response seen on EMB, the resolution of which was associated with reduced edema (and thus hypertrophy) and improvement in LV function.
Extrapulmonary manifestations of legionellosis are uncommon; the heart, particularly the myocardium, is the most usual site.8 Serogroup 1 is identified in most cases and a positive urine antigen test is diagnostic, with sensitivity of 90% and specificity of 100%.8
The first case of myocarditis associated with Legionella pneumonia was described in 1981 by Gross et al.,9 and 10 cases had been reported in the English-language literature by 2012.8 Myocarditis caused by Legionella is rare in adults, but in 2009 Burke et al.10 reported a case of L. pneumophila-induced perimyocarditis in the absence of pneumonia in a 50-year-old woman.
The prognosis of Legionella myocarditis depends on systemic involvement, comorbidities and the delay before beginning directed therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Damásio AF, Rodrigues L, Miranda L, et al. Miocardite fulminante a Legionella pneumophila – a propósito dum caso clínico. Rev Port Cardiol. 2014;33:185.e1–185.e5.