Heart rate recovery, defined as the fall in heart rate during the first minute after exercise, is an indicator of autonomic function, and has been found to be an independent predictor of mortality after acute myocardial infarction. Exercise training has several well-known benefits in terms of cardiorespiratory fitness, modifiable cardiovascular risk factors and prognosis after acute coronary events. However, there are no randomized controlled studies in the literature evaluating the effects of exercise training per se, controlling for changes in medication and diet, on heart rate recovery. Thus, this study aims to assess the effects of exercise training on autonomic function in coronary artery disease patients recovering from acute myocardial infarction.
MethodsThirty-eight patients following a first acute myocardial infarction participated in this prospective randomized clinical trial. Patients were randomized into two groups: exercise training or control. The exercise group participated in an 8-week aerobic exercise program, while the control received standard medical care and follow-up. Changes in hemodynamics at rest and at peak exercise (heart rate, systolic and diastolic blood pressure, and rate pressure product), dietary intake, cardiorespiratory fitness, and heart rate recovery were assessed.
ResultsMedication and diet remained unchanged in both groups during the study period. The exercise-training group improved resting hemodynamics, particularly resting heart rate (from 68.0±9.2 to 62.6±8.7bpm, p=0.030) and systolic blood pressure (from 135±7.1 to 125.6±11.3mmHg, p=0.012), cardiorespiratory fitness (from 30.8±7.8 to 33.9±8.3ml/min/kg, p=0.016), and heart rate recovery (from 20±6 to 24±5bpm, p=0.007). No significant changes were observed in the control group.
ConclusionsExercise training improved autonomic function, assessed by heart rate recovery, resting heart rate and systolic blood pressure, in the absence of changes in diet or medication.
A frequência cardíaca de recuperação, definida como a diminuição na frequência cardíaca durante o primeiro minuto após a cessação do exercício, é indicador da função autonómica e tem sido descrito como preditor independente de mortalidade após enfarte agudo do miocárdio. Os benefícios do exercício regular na capacidade cardiorrespiratória, nos factores de risco tradicionais modificáveis e no prognóstico após evento coronário agudo são bem conhecidos. Contudo, continua a faltar na literatura um estudo randomizado, controlado, avaliando os efeitos do exercício regular per se, controlando para alterações na medicação e dieta, na frequência cardíaca de recuperação. Desta forma, este estudo tem por objectivo avaliar o efeito de um programa de exercício físico na função autonómica em doentes com doença coronária após enfarte agudo do miocárdio.
MétodosParticiparam neste estudo prospectivo, randomizado, controlado 38 doentes após o primeiro enfarte agudo do miocárdio. Os doentes foram divididos em 2 grupos: grupo de exercício e grupo controlo. O grupo de exercício participou num programa de exercício aeróbio com 8 semanas de duração, enquanto o grupo controlo recebeu os cuidados e o seguimento médico habitual. Foram avaliadas as alterações nos parâmetros hemodinâmicos em repouso e no pico de exercício (frequência cardíaca, pressão arterial sistólica e diastólica e duplo produto), dieta, capacidade cardiorespiratória e frequência cardíaca de recuperação.
ResultadosDurante o estudo a medicação e a dieta não sofreram alterações em ambos os grupos. O grupo de exercício melhorou os parâmetros hemodinâmicos em repouso, nomeadamente a frequência cardíaca (68.0±9.2 para 62,6±8,7 bpm, P=0,030) e a pressão arterial sistólica (135±7.1 para 125,6±11.3mmHg, P=0,012), a capacidade cardiorespiratória (30,8±7,8 para 33,9±8,3ml/min/kg, P=0,016) e frequência cardíaca de recuperação (20±6 para 24±5 bpm, P=0,007). Não se observaram diferenças significativas no grupo controlo.
ConclusõesO programa de exercício físico melhorou a função autonómica, avaliada através da frequência cardíaca de recuperação, a frequência cardíaca e a pressão arterial de repouso mesmo na ausência de alterações na dieta e na medicação.
Dysfunction of the autonomic nervous system appears to be implicated in the pathophysiology of coronary artery disease (CAD) and is associated with increased risk of morbidity and mortality.1–3 Autonomic function can be evaluated by measuring resting heart rate (HR), heart rate variability, or heart rate recovery (HRR) following exercise.2,3 The effect of exercise training on autonomic function in CAD patients has also been assessed using these indicators.4–6 Nevertheless, measurement of HR variability requires sophisticated software, is not intuitive for most clinicians, and is difficult to implement on a routine clinical basis.2 In this context, exercise HRR has emerged as probably the simplest method of assessing autonomic function, particularly parasympathetic tone.3
HRR, defined as the drop in HR during the first minute immediately after exercise, is a strong predictor of mortality in CAD patients,7 independent of exercise capacity, left ventricular function, angiographic severity of CAD, myocardial perfusion defects, and changes in HR during exercise.7,8 Exercise-based cardiac rehabilitation has been associated with several positive effects on CAD patients,9 including improvements in HRR.4–6,10 However, these were not randomized controlled studies,4–6,10 evaluate patients with different CAD presentations,4,5 and used different methodologies to assess HRR. For instance, one study assessed HRR after submaximal exercise and a 1-min cool-down.4 The purpose of the present study was thus to evaluate the effects of exercise training on autonomic function in CAD patients recovering from acute myocardial infarction.
MethodsStudy design, sample size and powerThe required sample size for the present single-center prospective, randomized, controlled, parallel-group study was computed in advance based on the effect size calculated from previous studies.4,6 The statistical power for the matched pairs analysis revealed that 16 patients per group were required to detect a large effect size (0.9) in HRR with a power of 90% and a two-sided 5% significance level. A target of 24 patients per group was identified to accommodate an expected maximum dropout rate of 30%.
PatientsForty-seven consecutive CAD patients referred to the Cardiology Department with acute myocardial infarction participated in this study. Exclusion criteria were: recurrent acute myocardial infarction, unstable angina, reduced left ventricular function, pulmonary and renal comorbidities, and myocardial ischemia and/or severe ventricular arrhythmias during baseline exercise testing. Additionally, patients who changed medication during the study and those in the exercise-training group who failed to attend at least 80% of the exercise sessions were excluded from the statistical analysis. The ethics committee of the hospital approved the study. Written informed consent was obtained and all procedures were conducted in accordance with the Declaration of Helsinki.
Procedures and measurementsFrom inspection of their medical records, potential participants in the study were asked to come to the hospital on two different days one month after hospital discharge. On the first day, blood was collected for determination of lipid profile and levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) after an overnight fast of at least 12h, and compliance with the prescribed medication was monitored by a cardiologist and recorded in the medical record. Lipid profile was determined by enzymatic methods (Synchron LX 20 Analyzer, Beckman Coulter Inc., Fullerton, CA, USA). Plasma NT-proBNP levels were measured by means of a 1-step electrochemiluminescence enzyme immunoassay (Elecsys, Roche Diagnostics, Mannheim, Germany).
On the second day (2–3 days after the first visit), patients underwent the following evaluations: resting hemodynamics (HR, systolic and diastolic blood pressure), and a maximal or symptom-limited exercise testing to assess cardiorespiratory fitness, hemodynamics at peak exercise (peak HR, peak systolic and diastolic blood pressure), and HRR at first minute.
Resting systolic and diastolic blood pressure and HR were measured using a digital automatic blood pressure monitor (Omron M6 Comfort, Omron Healthcare Co., Ltd., Kyoto, Japan) with the patient seated, the right arm resting on a table so the brachial artery was level with the heart. Two measurements were then obtained at 1-min intervals and their mean was recorded. For systolic blood pressure, if there was more than 5mmHg difference between the two readings, one more reading was obtained for the mean. Resting rate-pressure product was computed by multiplying resting HR by resting systolic blood pressure.
Maximal or symptom-limited treadmill exercise testing was conducted according to the modified Bruce protocol. Pulmonary gas exchange analysis was performed throughout the test (Cardiovit CS-200 Ergo-Spiro, Schiller, Baar, Switzerland). Measurement of oxygen uptake (VO2), carbon dioxide production, minute ventilation, and respiratory exchange ratio were collected breath-by-breath. Peak VO2 was defined as the highest VO2 achieved by the patient during the test. Values for VO2 were indexed to body weight. Using 12-lead electrocardiogram readings, HR was monitored during the test and averaged every 10s. Peak HR was considered the highest mean 10-s value achieved during the test. Blood pressure was measured with a mercury sphygmomanometer at rest, during the last 45s of each stage of exercise, and in the last 15s of exercise nearing the end of the test. Peak systolic and diastolic blood pressures were recorded as the highest value achieved during the test. Peak rate-pressure product was computed by multiplying peak HR by peak systolic blood pressure. HRR was determined by calculating the difference between HR at peak exercise and HR at 1min after completion of exercise.10 Patients were instructed to sit after ending the test, and there was no cool-down period until after HRR was recorded.11 There was then a 4-min cool-down period that consisted of light walking (2km/h at 0% grade).
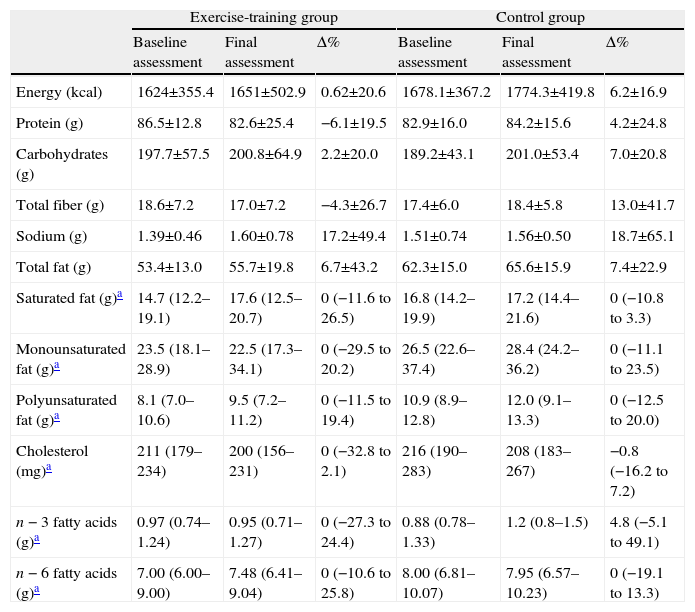
Dietary intake was evaluated by the participants completing a four-day food diary, providing details about their diet on four consecutive days including Sunday following the second visit according to previously described methodology.12 Nutrient intake data were obtained by multiplying the frequency of consumption of each food item by the nutrient content of the specified portion size, with once a day equaling one. For detailed nutrient analysis, the Food Processor Plus Program version 7.02 (ESHA Research, Salem, OR, USA), based on values from the US Department of Agriculture, was used. Additionally, values for typical Portuguese foods were computed using the Portuguese tables of food composition, typical recipes, and data from previous studies.12 Dietary intake was summarized in the following parameters: total energy intake, and consumption of protein, carbohydrates, total fiber, sodium, total fat, saturated fat, monounsaturated fat, cholesterol, and n−3 and n−6 fatty acids.
Following the baseline assessments, patients were randomly assigned to an exercise-training group (n=24) or a control group (n=23). Complete randomization was performed by allowing the patients to choose one of two sealed numbered envelopes containing the allocation to the groups. The exercise-training group participated in an 8-week outpatient exercise-training program, while the control group received only standard medical care and follow-up. Standard care and follow-up consisted of regular appointments with a cardiologist and medication. No advice regarding any form of exercise was provided to the control group. After the completion of the 8-week period and within no more than 4–5 days, patients of both groups underwent the final assessment, which included the same procedures performed at baseline. All the assessments were performed in the morning by the same examiners.
Exercise-training programPatients in the exercise-training group participated in a supervised outpatient exercise-training program. The exercise training was performed on three days per week for eight weeks. Each exercise session included 10min of warm-up, 35min of aerobic exercise, and 10min of cool-down. The exercise intensity was calculated as 65–75% of maximal HR achieved in treadmill exercise testing, in accordance with the guidelines.13 Individualized exercise prescription was periodically adjusted to encourage a gradual increase in overall exercise performance. In addition to the supervised exercise sessions, each patient was encouraged to exercise daily outside the formal exercise program.
Data analysisThe data were analyzed using SPSS 17.0 and are reported as absolute values and as percentage change ([final value−baseline value/baseline value]*100). Normally distributed variables are reported as mean±SD, and those that were not are reported as median (25th–75th percentile). The Student's independent t-test, the Mann–Whitney U test and the chi-square test were used for comparisons between groups in normally distributed, not normally distributed, and nominal data, respectively. The Student's paired t-test and the Wilcoxon signed-rank test were performed for comparisons within groups for normally and not normally distributed data, respectively. Additionally, each group was further divided into two subgroups on the basis of baseline blood pressure (pre-hypertension/hypertension versus normotension) in order to assess the influence of the baseline values on the effects of exercise training on blood pressure. The criteria to define the subgroups were systolic blood pressure>120mmHg and/or diastolic blood pressure>80mmHg to define pre-hypertension/hypertension.14 The level of statistical significance was p<0.05.
ResultsOf the forty-seven patients who began the study, seven withdrew prematurely (two moved house, one died, one broke his leg, and three for no specified reason) and two were excluded from the statistical analysis because they only participated in 50% of the exercise sessions. Thus, 20 patients in the exercise training group and 18 in the control group were included in the statistical analysis.
The time from hospital admission with acute myocardial infarction and the beginning of the study (exercise training or control period) was 40±4 days.
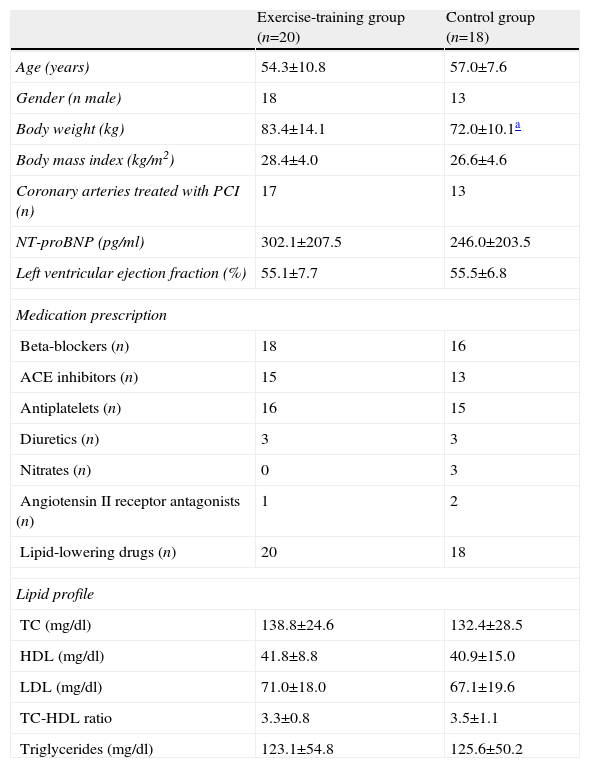
Patients’ general characteristics at baseline were similar in both groups (Table 1). Medication (Table 1) and diet (Table 2) remained unchanged during the study period in both groups.
Patients’ baseline characteristics.
| Exercise-training group (n=20) | Control group (n=18) | |
| Age (years) | 54.3±10.8 | 57.0±7.6 |
| Gender (n male) | 18 | 13 |
| Body weight (kg) | 83.4±14.1 | 72.0±10.1a |
| Body mass index (kg/m2) | 28.4±4.0 | 26.6±4.6 |
| Coronary arteries treated with PCI (n) | 17 | 13 |
| NT-proBNP (pg/ml) | 302.1±207.5 | 246.0±203.5 |
| Left ventricular ejection fraction (%) | 55.1±7.7 | 55.5±6.8 |
| Medication prescription | ||
| Beta-blockers (n) | 18 | 16 |
| ACE inhibitors (n) | 15 | 13 |
| Antiplatelets (n) | 16 | 15 |
| Diuretics (n) | 3 | 3 |
| Nitrates (n) | 0 | 3 |
| Angiotensin II receptor antagonists (n) | 1 | 2 |
| Lipid-lowering drugs (n) | 20 | 18 |
| Lipid profile | ||
| TC (mg/dl) | 138.8±24.6 | 132.4±28.5 |
| HDL (mg/dl) | 41.8±8.8 | 40.9±15.0 |
| LDL (mg/dl) | 71.0±18.0 | 67.1±19.6 |
| TC-HDL ratio | 3.3±0.8 | 3.5±1.1 |
| Triglycerides (mg/dl) | 123.1±54.8 | 125.6±50.2 |
ACE: angiotensin-converting enzyme; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PCI: percutaneous coronary intervention; TC: total cholesterol.
Changes in dietary intake in both groups.
| Exercise-training group | Control group | |||||
| Baseline assessment | Final assessment | Δ% | Baseline assessment | Final assessment | Δ% | |
| Energy (kcal) | 1624±355.4 | 1651±502.9 | 0.62±20.6 | 1678.1±367.2 | 1774.3±419.8 | 6.2±16.9 |
| Protein (g) | 86.5±12.8 | 82.6±25.4 | −6.1±19.5 | 82.9±16.0 | 84.2±15.6 | 4.2±24.8 |
| Carbohydrates (g) | 197.7±57.5 | 200.8±64.9 | 2.2±20.0 | 189.2±43.1 | 201.0±53.4 | 7.0±20.8 |
| Total fiber (g) | 18.6±7.2 | 17.0±7.2 | −4.3±26.7 | 17.4±6.0 | 18.4±5.8 | 13.0±41.7 |
| Sodium (g) | 1.39±0.46 | 1.60±0.78 | 17.2±49.4 | 1.51±0.74 | 1.56±0.50 | 18.7±65.1 |
| Total fat (g) | 53.4±13.0 | 55.7±19.8 | 6.7±43.2 | 62.3±15.0 | 65.6±15.9 | 7.4±22.9 |
| Saturated fat (g)a | 14.7 (12.2–19.1) | 17.6 (12.5–20.7) | 0 (−11.6 to 26.5) | 16.8 (14.2–19.9) | 17.2 (14.4–21.6) | 0 (−10.8 to 3.3) |
| Monounsaturated fat (g)a | 23.5 (18.1–28.9) | 22.5 (17.3–34.1) | 0 (−29.5 to 20.2) | 26.5 (22.6–37.4) | 28.4 (24.2–36.2) | 0 (−11.1 to 23.5) |
| Polyunsaturated fat (g)a | 8.1 (7.0–10.6) | 9.5 (7.2–11.2) | 0 (−11.5 to 19.4) | 10.9 (8.9–12.8) | 12.0 (9.1–13.3) | 0 (−12.5 to 20.0) |
| Cholesterol (mg)a | 211 (179–234) | 200 (156–231) | 0 (−32.8 to 2.1) | 216 (190–283) | 208 (183–267) | −0.8 (−16.2 to 7.2) |
| n−3 fatty acids (g)a | 0.97 (0.74–1.24) | 0.95 (0.71–1.27) | 0 (−27.3 to 24.4) | 0.88 (0.78–1.33) | 1.2 (0.8–1.5) | 4.8 (−5.1 to 49.1) |
| n−6 fatty acids (g)a | 7.00 (6.00–9.00) | 7.48 (6.41–9.04) | 0 (−10.6 to 25.8) | 8.00 (6.81–10.07) | 7.95 (6.57–10.23) | 0 (−19.1 to 13.3) |
Δ%: percentage change.
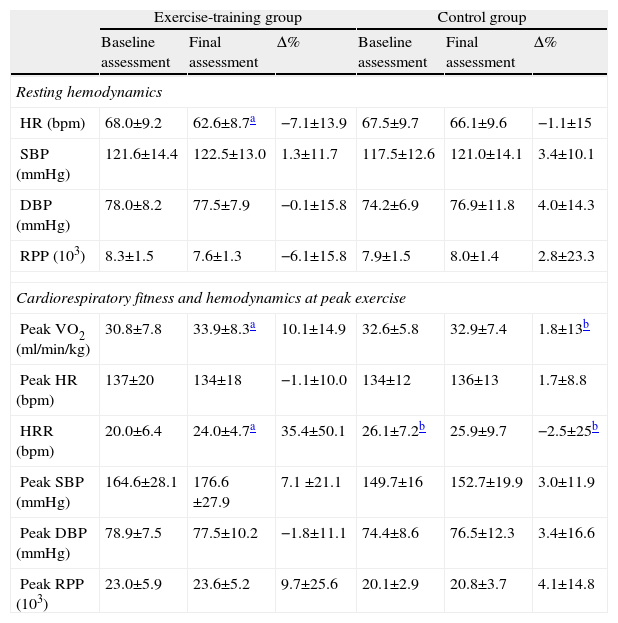
Resting HR decreased by 5.5bpm in the exercise-training group (p=0.03, Table 3). Resting and peak rate-pressure product and systolic and diastolic blood pressure were not changed by exercise training (Table 3).
Changes in hemodynamics at rest and at peak exercise, cardiorespiratory fitness.
| Exercise-training group | Control group | |||||
| Baseline assessment | Final assessment | Δ% | Baseline assessment | Final assessment | Δ% | |
| Resting hemodynamics | ||||||
| HR (bpm) | 68.0±9.2 | 62.6±8.7a | −7.1±13.9 | 67.5±9.7 | 66.1±9.6 | −1.1±15 |
| SBP (mmHg) | 121.6±14.4 | 122.5±13.0 | 1.3±11.7 | 117.5±12.6 | 121.0±14.1 | 3.4±10.1 |
| DBP (mmHg) | 78.0±8.2 | 77.5±7.9 | −0.1±15.8 | 74.2±6.9 | 76.9±11.8 | 4.0±14.3 |
| RPP (103) | 8.3±1.5 | 7.6±1.3 | −6.1±15.8 | 7.9±1.5 | 8.0±1.4 | 2.8±23.3 |
| Cardiorespiratory fitness and hemodynamics at peak exercise | ||||||
| Peak VO2 (ml/min/kg) | 30.8±7.8 | 33.9±8.3a | 10.1±14.9 | 32.6±5.8 | 32.9±7.4 | 1.8±13b |
| Peak HR (bpm) | 137±20 | 134±18 | −1.1±10.0 | 134±12 | 136±13 | 1.7±8.8 |
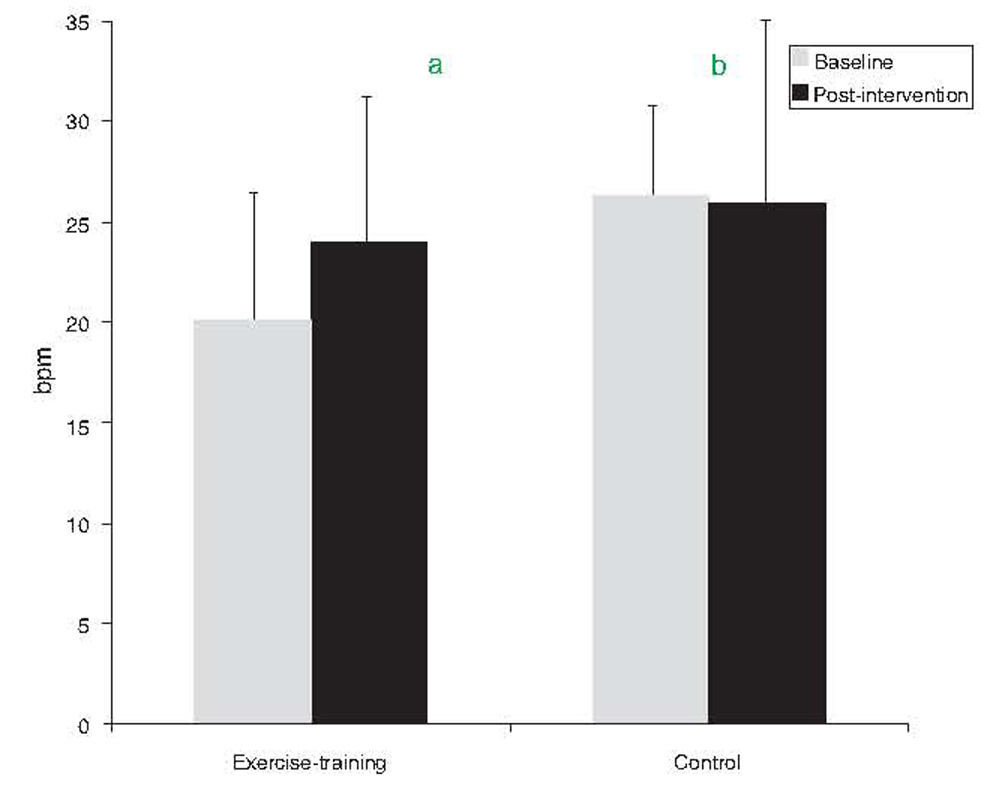
| HRR (bpm) | 20.0±6.4 | 24.0±4.7a | 35.4±50.1 | 26.1±7.2b | 25.9±9.7 | −2.5±25b |
| Peak SBP (mmHg) | 164.6±28.1 | 176.6±27.9 | 7.1 ±21.1 | 149.7±16 | 152.7±19.9 | 3.0±11.9 |
| Peak DBP (mmHg) | 78.9±7.5 | 77.5±10.2 | −1.8±11.1 | 74.4±8.6 | 76.5±12.3 | 3.4±16.6 |
| Peak RPP (103) | 23.0±5.9 | 23.6±5.2 | 9.7±25.6 | 20.1±2.9 | 20.8±3.7 | 4.1±14.8 |
DBP: diastolic blood pressure; HR: heart rate; HRR: heart rate recovery; RPP: rate-pressure product; SBP: systolic blood pressure; Δ%: percentage change.
Of the total of 38 patients that composed the two groups, five in the control group and nine in the exercise-training group presented levels of blood pressure classified as pre-hypertensive or hypertensive despite being medicated. In these patients, exercise training significantly decreased systolic (135±7.1–125.6±11.3mmHg, p=0.012), but not diastolic blood pressure (80.6±10.1–77.2±9.4mmHg; p=0.360). In the control group, both systolic (134±5.5–130±14.1mmHg, p=0.477) and diastolic (from 76±5.5–85±15.8mmHg, p=0.181) blood pressure remained unchanged.
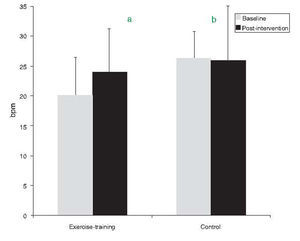
After exercise training an 8.5% improvement in peak VO2 was observed (p=0.016). By contrast, peak VO2 did not change in the control group (Table 3). Regarding HRR after exercise, at baseline the control group showed greater recovery than the exercise-training group (26±7 vs. 20±6bpm, p=0.008). The exercise-training program induced a significant increase in recovery at 1min, from 20±6 to 24±5bpm (p=0.007), whereas the control group remained unchanged (Figure 1). The percentage change confirmed the positive effect of exercise training, the change in HRR from baseline to the end of the study being significantly different between the groups (35.4±50.1 vs. −2.5±25%, p=0.009).
DiscussionThe present study aimed to assess the effects of exercise training on autonomic nervous function, with HRR as the main indicator, in a group of CAD patients recovering from a first acute myocardial infarction. To determine the effect of exercise training per se other important variables were controlled such as medication and diet. The main finding of our study is that exercise training improves autonomic function independently of medication or diet.
The positive effect of exercise training on the autonomic nervous system suggested above is supported by the faster HRR observed in the exercise-training group. HRR is an indirect and simple marker of autonomic function based on the decline of HR after acute exercise.2,3 In apparently healthy subjects and in athletes, HR rapidly falls after exercise termination.15 During a graded exercise test, HR increases as a result of withdrawal of parasympathetic tone and increased sympathetic tone.15 Immediately after exercise, HR decreases rapidly, mainly due to rapid reactivation of the parasympathetic nervous system.15 This ability of HR to recover following exercise, associated with the capacity of the cardiovascular system to reverse autonomic nervous system and baroreceptor adaptations, is often termed vagal reactivation.15,16
As in previous studies,10,17,18 we observed an improvement in HRR after exercise training. For instance, Giallauria et al.10 reported that HRR at 1min post-exercise improved by approximately 6bpm after 3 months of exercise in patients after myocardial infarction; also, Streuber et al.17 described an improvement of 5bpm after 12 weeks of exercise in heart failure patients. Faster HRR is of particular interest, since the rate of recovery following exercise has been shown to be inversely associated with the occurrence of cardiac events and all-cause mortality.7,8
Exercise training also decreased patients’ resting HR by 7.1%. This effect may be associated with increased parasympathetic nervous system tone and/or decreased sympathetic drive induced by exercise training.2 In CAD patients, exercise training has been shown to increase resting arterial baroreflex sensitivity,19 to decrease muscle sympathetic nerve activity,19 and to reduce circulating catecholamine levels.20 This decrease in resting HR also has positive prognostic value; pathophysiological studies indicate that high resting HR is associated with various detrimental effects, for instance on the stability of pre-existing atherosclerotic plaques.21 Nevertheless, it may be asked whether decreasing HR from 68 to 62bpm is clinically as well as statistically significant. We believe so, since a parallel increase in cardiovascular risk with HR was recently reported, at least for values above approximately 60bpm.22
Since diet, which at baseline was close to that recommended in the guidelines,23 and medication remained unchanged, the positive effects observed on blood pressure of patients with blood pressure above that recommended must have been the result of exercise training. Our results are in agreement with an elegant meta-analysis evaluating the effectiveness of exercise-based rehabilitation in CAD patients that reported a mean reduction of 3.2mmHg in systolic blood pressure.24
The effects of exercise on resting blood pressure can be explained by enhanced endothelium-dependent vasodilatation resulting from increased nitric oxide bioavailability, decreased endothelin-1 concentrations, and changes in the angiotensin receptor system.25–27
The patients in our study were under the medication recommended by the international guidelines, and the majority of the measured parameters were below recommended levels at baseline, which limits generalization of our findings to high-risk cardiac patients. Thus, further randomized controlled studies are needed to ascertain the effects of exercise training in patients with impaired HRR at baseline.
ConclusionsIn conclusion, exercise training improved autonomic function, as indicated by improvements in HRR, resting HR and systolic blood pressure. The clinical messages of the present study are that (i) standard care and follow-up were shown to be inadequate to improve autonomic function, and (ii) even patients with a good baseline profile after acute myocardial infarction showed considerable improvements with exercise, justifying referral for exercise training programs.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) Grant SFRH/BD/15843/2005 to the first author.