Stem cell therapy and aerobic exercise are non-pharmacological therapies following myocardial infarction. The aim of this study was to test whether aerobic exercise training enhances the benefits of mesenchymal stem cell (MSC) therapy on remodeling of the extracellular matrix and fetal gene expression in the left ventricle of infarcted rats.
MethodsMyocardial infarction was surgically induced in six-week old male Wistar rats. Animals were divided into four groups: sedentary control (SC) and sedentary and stem cell treated (SCMSC); exercised (EX) and exercised and stem cell treated (EXMSC). Bone marrow-derived MSCs were immediately transplanted via the tail vein (concentration: 1×106 cells). Exercise training (five days/week, 60 min/day; 60% of maximal running speed) started 24 hours after myocardial infarction and lasted for 12 weeks.
ResultsExercise capacity was higher in exercised than in sedentary groups. Animals in the SCMSC, EX and EXMSC groups exhibited better cardiac function than those in SC. Collagen content was lower in the SCMSC, EX and EXMSC groups than in SC and skeletal α-actin expression was lower in EX and EXMSC than in SC. The α/β-MHC ratio was higher in EX and EXMSC than in SC. The combination of therapies further reduced collagen content in the remote region of the infarct (∼24%) and skeletal α-actin expression (∼30%).
ConclusionAerobic exercise training appears to enhance the beneficial effects of stem cell therapy on remodeling of the extracellular matrix and fetal gene expression in the left ventricle of rats with moderate infarction.
Terapia com células estromais e exercício aeróbio são estratégias não farmacológicas no tratamento pós-enfarte do miocárdio. O objetivo foi testar se o treinamento aeróbio amplia os benefícios da terapia com células mesenquimais estromais (CME) na remodelagem da matriz extracelular e na expressão de genes fetais no ventrículo esquerdo de ratos enfartados.
MétodosO enfarte do miocárdio foi induzido cirurgicamente em ratos com seis semanas de idade, divididos em quatro grupos: sedentário controle (SC) e tratado com CME (SCCME); exercício (EX) e exercício tratado com CME (EXCME). CME derivadas da medula óssea foram transplantadas através da veia caudal (concentração: 1 x 106 células). O treinamento aeróbio (5 dias/semana; 60min/dia; 60% da velocidade máxima de corrida) iniciou 24h após enfarte do miocárdio e durou 12 semanas.
ResultadosA capacidade de exercício foi maior nos grupos exercitados que nos sedentários. Os grupos SCCME, EX e EXCME apresentaram melhor função cardíaca do que o SC. O conteúdo de colagénio foi menor nos grupos SCCME, EX e EXCME que no SC. A expressão da α-actina esquelética foi menor nos grupos EX e EXCME, comparada à do SC. A razão miosina de cadeia pesada α/β foi maior nos grupos EX e EXCME, comparada à do SC. A combinação das terapias reduziu ainda mais o conteúdo de colagénio na região remota (∼24%) e a expressão de α-actina esquelética (∼30%).
ConclusãoO treinamento aeróbio parece potencializar os benefícios da terapia com células mesenquimais estromais na remodelagem da matriz extracelular do ventrículo esquerdo e expressão de genes fetais em ratos com enfarte moderado.
Left ventricular (LV) remodeling after myocardial infarction (MI) is one of the most common causes of heart failure (HF), and correlates directly with ventricular dysfunction and increased morbidity and mortality.1 HF is characterized by cardiomyocyte death or hypertrophy, collagen accumulation and molecular changes, such as re-expression of fetal genes including skeletal α-actin and beta-myosin heavy chain (β-MHC) in the heart.2 The use of non-pharmacological therapies is known to improve cardiac function, quality of life and survival rates in patients with post-infarction HF.3,4
One such approach is mesenchymal stem cell (MSC) therapy. This therapy is associated with reduced myocardial infarct size, reduced LV wall thinning, antiapoptotic effects and attenuation of ventricular remodeling by antifibrotic action. Moreover, MSC therapy improves myocardial perfusion and preserves systolic and diastolic performance as well as cardiac electrical viability and impulse propagation.5–8 Aerobic exercise training has also been shown to attenuate HF symptoms and to improve systolic and diastolic function and cellular contraction, as well as presenting a pattern of cardiac gene expression that is distinct from that of pathological cardiac adaptation.9–11
The effects on infarcted hearts of combining MSC therapy and aerobic exercise training, however, are not yet clear.12 Recently, our group showed that early exercise training and MSC therapy proved beneficial to LV morphology and function and to myocyte contractility in infarcted rats, although a beneficial effect of combining these therapies was not seen.13 Nevertheless, adaptations of the LV extracellular matrix and fetal gene re-expression to this combination of therapies have not been elucidated. Thus, the aim of this study was to test whether aerobic exercise training enhances the benefits of stem cell therapy on remodeling of the extracellular matrix and fetal gene expression in the left ventricle of infarcted rats.
MethodsExperimental animalsSix-week-old male Wistar rats (body mass 120 g) were kept in cages under a 12:12-hour light-dark cycle in a temperature-controlled room (22°C), with free access to water and standard rodent diet. Myocardial infarction was surgically induced in 30-day-old rats (118 g). The animals were anesthetized with 3% isoflurane and 100% oxygen at a constant flow rate of 1 ml/min, intubated, and ventilated. After extensive thoracotomy, ligation of the left anterior descending (LAD) coronary artery was performed, as described previously.1 The animals were then randomly divided into groups by simple random sampling: sedentary control (SC), sedentary treated with MSCs (SCMSC), exercised (EX), and exercised treated with MSCs (EXMSC). All groups began the experimental period with eight animals, however during MI induction some animals were lost. Thus, the final number of animals in each group is specified in the figures and tables.
Experiments were conducted in accordance with the Care and Use of Laboratory Animals, 2011, and were approved by the local Ethics Committee for Animal Use (CEUA-UFV Protocol No. 05/2013).
Stem cell therapyMSCs collected from the femur marrow of five male Wistar rats (age six weeks) were isolated, expanded and maintained in culture, and characterized by flow cytometry to confirm cell type, as previously described.13 Aliquots of 1×106 cells were prepared in 1.0 ml phosphate-buffered saline (PBS) and injected via the tail vein immediately after MI. Animals not treated with MSC (SC and EX groups) were injected with the same dosage of PBS only.
In order to identify transplanted MSCs in the rats, five extra animals were injected with the same dose of MSCs labeled using the Qtracker® 655 Cell Labeling Kit (Invitrogen) according to the manufacturer's recommendations. These animals were euthanized 18 hours after MI and fragments of heart, spleen, and lung were histologically analyzed, as described previously.13 Labeled stem cells were found in the lung tissue of the five infarcted animals, however no sign of labeled stem cells was observed in the heart and spleen tissue of these animals.
Exercise training and maximal running speed protocolsAnimals from the EX and EXMSC groups underwent a 12-week treadmill exercise training program. One week before MI all animals were adapted to the treadmill by running 5 min/day, 5 m/min, for five days. The exercise training program was begun 24 hours after MI. During the first week rats in the EX and EXMSC groups ran 10 min/day, 0% grade, at a speed of 7-10 m/min, and the duration of exercise progressed from 10 to 50 min. To determine the maximal running speed by which to monitor running speed during the following exercise training sessions, at the beginning of the second week each animal in the EX and EXMSC groups underwent a progressive treadmill test until fatigue, as previously described.13 Then, from the second week onward, the running speed of the exercise training sessions was set at 60% of the mean maximal running speed obtained for each group, and the session duration was set at 60 min for both groups. The progressive treadmill test was repeated at the end of the fourth and eighth weeks to adjust the running speed of each group, and at the end of the twelfth week to assess the final exercise capacity of all experimental animals. The mean distance run by each group was adopted as the index of exercise capacity. All progressive treadmill tests were performed 24 hours after the last training session in the exercised groups.
Echocardiographic and electrocardiographic measuresAt the end of the experiment, 24 hours after the last progressive treadmill test, echocardiographic and electrocardiographic measures were performed as previously described.13,14 Animals were anesthetized in an induction chamber flushed with 1-1.5% isoflurane and 100% oxygen at a constant flow of 1 l/min. Once unconscious, they were placed on a platform in dorsal recumbency, with the four limbs fixed. Isoflurane was maintained at a sufficient concentration to restrain (0.5-1.0%), and the animals were able to maintain spontaneous breathing. These low concentrations of isoflurane were applied to immobilize the animal only for a short period for the examinations and are known to have no significant impact on cardiac function.
Echocardiographic exams included two-dimensional studies with a fast sampling rate of 120 fps and M-mode, using a MyLab™ 30 system (Esaote, Genoa, Italy) and phased array transducers with a nominal frequency of 11.0 MHz. Two-dimensional and M-mode transthoracic echocardiograms were obtained with a sweep speed of 200 mm/s and adjusted to heart rate according to the guidelines of the American Society of Echocardiography and stored for later analysis. Each parameter was measured in three different cardiac cycles and the mean of the measurements was used for statistical analysis. The M-mode recordings were analyzed by a blinded observer using an off-line analysis system available on the device itself.
For the electrocardiogram, lead II was recorded using the PowerLab data acquisition system (ADInstruments, São Paulo, Brazil), and data were analyzed with LabChart Pro (ADInstruments LabChart 7, São Paulo, Brazil). All measurements were obtained and analyzed by an experienced technician blinded to the study groups and treatments.
Euthanasia and sample collectionTwenty-four hours after echocardiographic and electrocardiographic examinations, and 12 weeks plus three days after MI, body mass was measured and the animal was euthanized by deepening anesthesia. Following thoracotomy, the heart was injected with potassium chloride (14 mM) and excised. After measurement of heart mass and volume, the atria were dissected away and the ventricles were cut transversely into three millimeter-thick rings (base, intermediate and apex), of which the intermediate ring was processed for histological analyses of infarct size and collagen content. One fragment from the remote area of infarction in the left ventricle was removed and stored at ?80°C for analysis of gene expression.
Determination of heart volumeAfter excision, heart volume was determined using the immersion method described by Scherle.15 Briefly, the heart was excised, dissected free of soft tissue, washed in saline solution and immersed in a beaker filled with saline solution. The volume of solution displaced was taken to be the heart volume, expressed in ml.
Infarct sizeInfarct size was measured as previously described.13 In summary, after dehydration and paraffin embedding, 5-μm thick transverse sections of the ventricles were obtained using a rotary microtome (Reichert-Jung 2045 Multicut, Heidelberg, Germany). Two histology slides per animal, each consisting of six sections, were mounted and stained with picrosirius red. A scanner (HP DeskJet F380, USA) was used to obtain images with a resolution of 600 dpi. Twenty images per infarcted group were obtained. The endocardial and epicardial circumferences of the infarcted tissue and of the left ventricle were determined with the aid of image analysis software (Image-Pro Plus 4.5; Media Cybernetics, Silver Spring, MD, USA). The following parameters were determined: circumference of endocardial scar (CENS); circumference of epicardial scar (CEPS); endocardial circumference of the left ventricle (ENCLV); and epicardial circumference of the left ventricle (EPCLV). Infarct size was expressed as a percentage using the following formula: (CENS + CEPS/ENCLV + EPCLV)×100 and calculated as the mean of 20 images per infarcted group.
Collagen contentCollagen content was assessed in two different regions of the left ventricle (transition and remote from the infarct). For this purpose, the same slides mounted to assess infarct size were used. For each region, 20 images per experimental group were captured using a SPOT Insight Color camera (Diagnostic Instruments, USA) coupled to a polarized light microscope (Olympus AX70®, Tokyo, Japan) in the 10× objective. The distribution of total collagen content was analyzed using Image Pro-Plus 4.5® image analysis software (Media Cybernetics, Silver Spring, MD, USA). In this analysis, 12 microscopic fields per region from six rats per group were randomly investigated. An 88-point grid was superimposed on each image, and a total of 1056 points per region were assigned for each rat. The area was determined by adding up these points, then dividing the result by the total points of the cross-section. The results are expressed as means ± standard error.
Fetal gene expressionMessenger RNA expression of alpha-myosin heavy chain (α-MHC), β-MHC and skeletal α-actin in LV tissue was determined using reverse transcription-polymerase chain reaction.16 Primer 3 software (http://frodo.wi.mit.edu/primer3/) was used to design the following primers: α-MHC (sense: 5′-CGA GTC CCA GGT CAA G-3′, antisense: 5′-AGG CTC TTT CTG GAC C-3′); β-MHC (sense: 5′-CAT CCC CAA TGA GAC GAA G-3′, antisense: 5′-AGG CTC TTT CTG GAC A-3′); skeletal α-actin (sense: 5′-ACC ACA GGC ATT GTT CTG GA-3′, antisense: 5′-TAA GGT AGT CAG TGA GGT CC-3′); and cyclophilin (sense: 5′-AAT GCT GGA CCA AAC ACA AA-3′, antisense: 5′-CCT TCT TTC ACC TTC CCA AA-3′). Genetic expression of cyclophilin served as a normalizer to obtain relative gene expression.
Statistical analysisData are presented as means ± standard error of the mean. A p-value <0.05 was considered statistically significant. Data were tested for normality by the Shapiro-Wilk test. Two-way analysis of variance, followed by Bonferroni's correction, was used to compare groups. The numbers of animals and images used in each experiment are given in the relevant table and figure legends.
ResultsThe general characteristics and exercise capacity of experimental rats are shown in Table 1. The exercise training applied increased total distance run and reduced resting heart rate in infarcted rats by itself and in combination with MSC, compared to sedentary controls. However, no effect of MCS therapy by itself was found. In addition, both MSC therapy and exercise training improved LV function in infarcted rats, increasing ejection fraction (EF) and fractional shortening (FS), and this effect was maintained when the therapies were combined. Regarding infarct size, MSC therapy and exercise training, both alone and combined, reduced infarct size, compared to the SC group; MSC therapy was more effective.
General characteristics, cardiac function and exercise capacity.
| SC | SCMSC | EX | EXMSC | |
|---|---|---|---|---|
| Distance run, m | 109.58±17.16 | 117.42±13.42 | 342.96±31.06a | 385.00±25.62a,b |
| RHR, bpm | 410.00±11.72 | 396.00±12.84 | 366.00±14.35a | 353.00±14.35a,b |
| FS, % | 21.51±1.41 | 28.35±1.60a | 28.11±1.73a | 27.38±0.99a |
| EF, % | 48.14±2.51 | 60.19±2.85a | 59.75±3.07a | 58.81±1.54a |
| Infarct size, % | 30.39±2.32 | 15.00±2.76a | 21.33±2.55a | 23.85±2.32a,b |
Data expressed as mean ± standard error of the mean of 6-8 animals in each group. Infarct size was calculated as the mean of 20 images in each group.
EF: ejection fraction; EX: exercised; EXMSC: exercised plus stem cells; FS: fractional shortening; RHR: resting heart rate; SC: sedentary control; SCMSC: sedentary control plus stem cells.
Body mass, heart mass and volume and morphometric data are presented in Table 2. Body mass, heart mass and the ratio between them, and heart volume were not affected by the therapies either alone or in combination. However, body mass tended to be lower in exercised animals (p=0.07). LV dimensions and posterior wall thickness in diastole did not differ between groups. LV posterior wall thickness in systole was greater in exercised animals than in the other groups.
Body mass, heart mass and volume, and morphometric data.
| SC | SCMSC | EX | EXMSC | |
|---|---|---|---|---|
| BM, g | 422.00±12.53 | 420.17±13.40 | 396.60±14.47 | 395.28±13.40 |
| HM, g | 1.81±0.07 | 1.91±0.08 | 1.70±0.09 | 1.76±0.08 |
| HM/BM | 0.43±0.23 | 0.46±0.23 | 0.43±0.23 | 0.44±0.21 |
| HV, ml | 1.13±0.06 | 1.08±0.06 | 1.16±0.06 | 1.17±0.06 |
| LVDD, mm | 8.13±0.30 | 7.91±0.35 | 7.54±0.37 | 8.12±0.35 |
| LVDS, mm | 6.44±0.31 | 5.68±0.36 | 5.42±0.38 | 5.89±0.36 |
| LVPWTd, mm | 1.86±0.11 | 1.85±0.12 | 2.12±0.13 | 1.88±0.12 |
| LVPWTs, mm | 2.51±0.12 | 2.69±0.14 | 3.30±0.15a | 2.73±0.14 |
Data expressed as means ± standard error of the mean of 6-8 animals in each group.
BM: body mass; EX: exercised; EXMSC: exercised plus stem cells; HM: heart mass; HV: heart volume; LVDD: left ventricular diastolic diameter; LVDS: left ventricular systolic diameter; LVPWTd: left ventricular posterior wall thickness in diastole; LVPWTs: left ventricular posterior wall thickness in systole; SC: sedentary control; SCMSC: sedentary control plus stem cells.
LV collagen content was positively altered by both therapies, both combined and in isolation (Table 3). Collagen content was reduced in the LV regions assessed in SCMSC (transition [∼40%]; remote [∼25%]) and in EX (transition [∼25%]; remote [∼25%]) compared to SC. The reduction in the transition region was also seen in EXMSC (combined effect). However, in the remote region, collagen content was further reduced in EXMSC (∼24%).
Collagen content in two regions of the left ventricle in infarcted rats.
| LV region | SC | SCMSC | EX | EXMSC |
|---|---|---|---|---|
| Transition, %/mm2 | 0.335±0.012 | 0.201±0.013a | 0.249±0.012a | 0.203±0.012a,b |
| Remote, %/mm2 | 0.086±0.006 | 0.064±0.007a | 0.064±0.006a | 0.044±0.007a |
Data expressed as means ± standard error of the mean of 72 images in each group.
EX: exercised; EXMSC: exercised plus stem cells; SC: sedentary control; SCMSC: sedentary control plus stem cells.
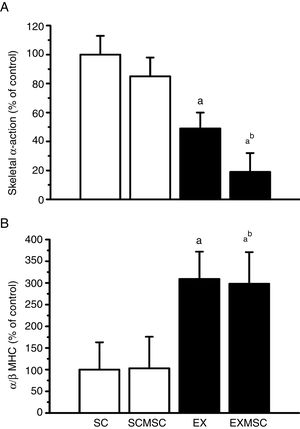
Data on fetal gene expression in the left ventricle are presented in Figure 1. Although no statistically significant effect of MSC therapy on skeletal α-actin expression was found, the exercise training regime used here reduced it considerably (∼50%). Moreover, the two therapies combined reduced it further (∼30%). Concerning α- and β-MHC expression, although MSC therapy had no effect in isolation, aerobic exercise training considerably increased the α/β-MHC ratio (∼200%). This benefit was maintained when these therapies were combined.
Gene expression in the remote area of the left ventricular infarct scar of infarcted rats. Data are means ± standard error of the mean of six animals in each group. α-MHC: alpha-myosin heavy chain; β-MHC: beta-myosin heavy chain; EX: exercised; EXMSC: exercised treated with stem cells; SC: sedentary control; SCMSC: sedentary treated with stem cells. a: p<0.05 vs. SC; b: p<0.05 vs. SCMSC.
Male Wistar rats with moderate-size infarcts (∼30%) had impaired LV function (i.e. reduced FS and EF in the SC group), mainly due to the loss of contractile tissue and subsequent reparative fibrosis (leading to increased collagen content), which together result in reduced cardiac compliance.2 In this setting, the left ventricle of infarcted animals also presented enhanced expression of skeletal α-actin and a high α/β-MHC ratio. Despite this, these animals responded positively to both the non-pharmacological therapies used here. Although there is disagreement concerning the efficacy of early and late interventions after MI using such therapeutic approaches,11,17,18 our results provide evidence of the benefits of early intervention.
Concerning the effects of MSC therapy in isolation, it improved LV function in infarcted rats, as shown by increased FE and FS. This adaptation may be due to enhanced impulse propagation and electrical conductivity in response to better preservation of cardiomyocytes and extracellular matrix in the infarcted heart.6 Moreover, MSC therapy also improves excitation-contraction coupling in infarcted hearts by modifying the Ca2+ transient and SERCA2a activity, resulting in better contractile function and preventing arrhythmias.5,13
Our results show that MSC therapy also reduced collagen content in the transition and remote regions of the infarct scar in the left ventricle of infarcted hearts. These findings are in agreement with other studies.7,8,19,20 MSCs are thought to act by releasing growth factors and cytokines that improve myocyte survival and cardiac remodeling. In addition, cell death by apoptosis and necrosis is reduced by MSC therapy, while angiogenesis and extracellular matrix remodeling are improved.21,22 Suggested mechanisms behind the reduction in collagen content include changes in matrix metalloproteinase expression and collagenase activity or other enzymatic pathways,8,22 and attenuation of collagen type I and III expression by inhibition of the proliferation of cardiac fibroblasts, thus exerting antifibrotic effects.11,20
Despite these benefits of MCS therapy on cardiac parameters, it did not result in improvement in the exercise capacity of infarcted rats. This indicates that the effect of MSC therapy on cardiac muscle was not sufficient to impact on exercise performance. It is also possible that our exercise test outcome (i.e. distance run) is more dependent on adaptations in peripheral and skeletal muscle than on those in cardiac muscle. Moreover, MSC therapy did not affect LV dimensions or posterior wall thickness.
Regarding aerobic exercise, rats subjected to exercise training 24 hours after MI induction exhibited improved physical capacity and reduced resting heart rate, and tended to have lower body mass. These parameters are established benefits of moderate-intensity aerobic exercise in infarcted animals.11,23,24 In line with these benefits, exercise training also increased FS and EF in infarcted rats. The main cellular mechanisms by which exercise improves cardiac function are increases in myocyte contractility, expression of Ca2+ regulatory proteins and/or activity, and sensitivity of myofilaments to Ca2+.9,13,25
The exercised rats also exhibited reduced LV collagen content. Aerobic exercise training begun one week after infarction has been shown to attenuate the action of tissue inhibitor of matrix metalloproteinase 1, which increases matrix metalloproteinase activity in infarcted rats.11 Thus, proteolytic activity may have increased, resulting in increased collagen degradation in our exercised animals. This adaptation is in line with the enhanced EF and FS detected in these rats, and reflects the improved cardiac complacency typical of endurance-trained hearts,18 although no effect of exercise on LV dimensions or posterior wall thickness in diastole was observed.
Moreover, our results showed that exercise training reduced the expression of skeletal α-actin and increased the α/β-MHC ratio in the left ventricle of infarcted animals. This result is contrary to that observed in infarcted rat hearts10 that exhibited elevated skeletal α-actin expression and reduced α/β-MHC ratio. Since the β-MHC isoform has fivefold lower ATPase activity than α-MHC, leading to decreases in contraction and relaxation velocities and poor energy utilization,26 the altered proportions of MHC isoforms (i.e. increased α/β MHC ratio) observed in our exercised animals helps to explain the enhanced cardiac function found in these rats. The exercise training used here also positively affected the genetic program of cardiac development after MI induction, as it reduced skeletal α-actin expression, a marker of pathological cardiac hypertrophy. Furthermore, we observed that exercise training increased LV posterior wall thickness in systole. These findings indicate that the exercise regime applied counteracted the deleterious fetal gene re-expression induced by MI and reinforces the idea that aerobic exercise training promotes beneficial gene adaptations that may be responsible, at least in part, for the improved cardiac function and exercise capacity in infarcted rats.
Our main interest here was in the effectiveness of aerobic exercise training in enhancing the effects of early MSC therapy. When early MSC therapy and aerobic exercise were combined (i.e. in the EXMSC group), some additional benefits were seen. For instance, in animals in that group, benefit was seen in the LV extracellular matrix, as the collagen content in the remote region of the infarct scar showed additional reduction (∼24%). Moreover, the expression of fetal genes was also positively affected. While the benefits of exercise training on α- and β-MHC expression were maintained, the expression of skeletal α-actin showed a further decrease (∼30%). In line with this effect, these animals had enhanced exercise capacity (∼30%) and LV posterior wall thickness in systole returned to control levels, though it was not reflected in additional gains in cardiac function or infarct size, as the benefits of the therapy in isolation remained the same or even decreased. Some synergy in the association of exercise training and cell transplantation in infarcted rats has been observed by others12 in a model of late interventions, but using different exercise regimes, stem cells and cell transplantation technique. It therefore appears that aerobic exercise can enhance the action of MSC therapy.
In our model, the MSCs injected into the tail vein were detected only in the lungs of the infarcted animals and not at the site of injury. This outcome has been reported elsewhere.27 Even so, we detected beneficial changes induced by MSC therapy in the infarcted hearts. Studies have shown that MSCs are not mere producers and mobile replacements, but secrete various factors, such as transforming growth factor-beta, prostaglandin E2, interleukin-10, programmed cell death ligand 1 and hepatocyte growth factor, that inhibit the immune response mediated by B and T lymphocytes, as well as natural killer cells, thus helping to reduce the inflammatory response caused by MI.28–30 Their immunomodulatory effect appears to be exerted locally in resident immune cells at the injury site as well as systemically.31 In addition, early transplantation after the ischemic event provides trophic support by stimulating angiogenesis and mitosis in specific tissue progenitor cells, and by inhibiting ischemia-induced apoptosis.32
ConclusionIn conclusion, aerobic exercise training appears to enhance the benefits of stem cell therapy on remodeling of the extracellular matrix and fetal gene re-expression in rats with moderate infarction. Together, these benefits improved the exercise capacity of infarcted rats.
Funding sourcesThis study was funded by CNPq (grant 473041/2012-0) and FAPEMIG (grant APQ01941-12). JS Freitas held a master's scholarship from CAPES. AJ Natali and EM de Oliveira are CNPq fellows.
Study associationThis article is part of the doctoral thesis by Juliana Silveira de Freitas at the Universidade Federal de Viçosa.
Conflicts of interestThe authors have no conflicts of interest to declare.