Cardiovascular diseases (CVD) are the most common cause of death worldwide. Among CVDs, heart failure (HF) is known to be the most common cause of hospitalization in patients over 65. Despite all proposed treatments for CVDs, mortality and morbidity still remain high. There are controversial reports available on vitamin D efficacy in patients with HF. In this systematic review and meta-analysis, we aimed to investigate whether vitamin D is effective at enhancing ejection fraction (EF) in patients with HF. We performed a systematic search across different databases (PubMed, SCOPUS, Web of Science, EMBASE, SciELO and Google Scholar) up to 1 Jan 2020 without any language or location limitations. Our suggested Population/Intervention/Comparison/Outcome/Type of study (PICOT) was P: patients with HF, I: vitamin D, C: placebo or no treatment, O: EF and T: clinical trials. To achieve the highest sensitivity, only keywords associate with P and I were selected for the search query. A total number of 5397 primary studies were found, of which 13 were elected for data synthesis. Clinical trials were published and available since 2005 up to 2019 and a total number of 1215 patients were included. Our results showed that vitamin D can significantly enhance left ventricular EF in HF patients by 3.304% (95% confidence interval [CI] 0.954, 5.654, p=0.006). Based on our observations, we conclude that before conducting a large number of high quality clinical trials and further meta-analysis, vitamin D should be prescribed to all patients with HF.
As doenças cardiovasculares (DCV) são a causa de morte mais comum em todo o mundo. De entre as DCV, a insuficiência cardíaca (IC) é a causa mais comum de hospitalização nos doentes com mais de 65 anos. Apesar de todos os tratamentos existentes para as DCV, as taxas de mortalidade e morbilidade permanecem muito altas. Existem relatórios controversos acerca da eficácia da vitamina D em doentes com IC. Neste estudo de revisão sistemática e meta-análise, propusemo-nos investigar se a vitamina D é ou não eficaz na melhoria da fração de ejeção (FE) em doentes com IC. Realizámos uma pesquisa sistemática em diferentes bases de dados (PubMed, Scopus, Web of Science, Embase, Sialo e Google Scholar) até 1 de janeiro de 2020 sem limitações de idioma ou de localização geográfica. O PICOT usado foi: P: doentes com IC, I: vitamina D, C: placebo ou sem tratamento, O: FE e T: ensaios clínicos. Para aumentar a sensibilidade, apenas palavras-chave associadas com O e I foram selecionadas para a pesquisa. Foram achados 5.397 estudos, dos quais apenas 13 foram selecionados para a síntese de dados. Os ensaios clínicos foram publicados entre 2005 e 2019 e 1215 doentes foram incluídos. Os nossos resultados mostraram que a vitamina D aumenta significativamente a FE em doentes com IC, em 3,304% [95% Intervalo de Confiança 0,954, 5,654] (valor-P=0,006). Com base nas nossas observações, sugerimos que, antes de realizar mais e melhores ensaios clínicos e ainda outras meta-análises, a vitamina D seja prescrita a todos os doentes com IC.
Cardiovascular diseases (CVD) are the most common cause of mortality and morbidity worldwide and lead to a high socio-economic burden on society. Despite the high mortality in CVDs, early diagnosis, careful prevention and treatment can reduce the global burden of CVDs significantly.1
Among the different etiologies of CVDs, heart failure (HF) is associated with very high mortality and morbidity, such as sudden cardiac death. HF is the most frequent cause of hospital admission in patients over 65 years. Considering universal increase in life expectancy, the number of patients diagnosed with HF is growing globally.2
In recent literature, different treatments are suggested for HF and there is agreement on the use of diuretics in many of them. At least small dosage of diuretics is prescribed for several patients with HF. Modern treatments for HF mostly target left ventricular systolic dysfunction and for this goal angiotensin converting enzyme inhibitors and beta-blockers are commonly used.3
Despite major improvements in the treatment of HF over the last three decades, hospitalization, mortality and morbidity rate still remain high. The effectiveness of vitamin D on different prognostic markers of HF has been investigated in studies with different designs. Controversial efficacy is reported for vitamin D in recent articles including primary research, review articles and even meta-analyses.4–7
In different articles, the importance of left ventricular ejection fraction (LVEF) is assessed in patients with HF. Heart failure is a pathological clinical condition defined by impaired pump function of the heart muscle, which is categorized by LVEF. Important prior researches, such as the CHARM trial,8 reported that lower LVEF is associated with higher cardiovascular death, mortality and rehospitalization rate9,10 in patients with HF.
There are several primary studies and a limited number of systematic reviews published regarding the efficacy of vitamin D supplementation in patients with HF and the results are controversial. There is no consensus among these papers and many of them have reported clearly opposing results. We therefore believe that an updated systematic review is required in this field.6,7,11
Considering the helpfulness of a cheap, accessible, and safe treatment such as vitamin D in clinical practice, that clinical trials are the best types of studies for decision-making and the major importance of LVEF index in estimating myocardial function in patients with HF; we focused specifically on the effect of vitamin D supplements on ejection fraction (EF) in a systematic review limited to clinical trials to achieve the greatest accuracy.
MethodsStudy design and search strategyThis is a systematic review and meta-analysis study performed on all clinical trials up to 1 Jan 2020 to assess the real clinical effect of vitamin D supplementation on LVEF in patients with HF. This study was designed and performed based on PRISMA guidelines.12 The protocol was prospectively registered with PROSPERO (international prospective register of systematic reviews of University of York, UK) under registration ID CRD42020175478.
In this systematic review and meta-analysis, we performed a very comprehensive search on PubMed, SCOPUS, Web of Science, EMBASE, SciELO and Google Scholar along with references of all included and related studies to achieve the best results.13 We also scanned systematic reviews in order to discover potential additional studies.
Our clinical question-based Population/Intervention/Comparison/Outcome/Type of study (PICOT) was: P: all patients with HF; I: vitamin D supplementation; C: placebo or no treatment; O: ejection fraction and T: clinical trials. While doing a primary search across databases we found that very limited number of clinical trials are published in this field and several desirable studies were discovered without the term “ejection fraction” in their topic so we designed a more sensitive and wider search with only keywords associated with “P” and “I” keywords.
Our PubMed search query was as: (((((((((((heart failure[Title/Abstract]) OR congestive heart failure[Title/Abstract]) OR cardiac failure[Title/Abstract]) OR chronic heart failure[Title/Abstract]) OR heart decompensation[Title/Abstract]) OR myocardial failure[Title/Abstract]) OR myocardial decompensation[Title/Abstract]) OR cardiac decompensation[Title/Abstract]) OR congestive cardiac failure[Title/Abstract]) OR heart failure[MeSH Terms])) AND ((((((((((((vitamin D[Title/Abstract]) OR cholecalciferol[Title/Abstract]) OR calcitriol[Title/Abstract]) OR hydroxycholecalciferols[Title/Abstract]) OR dihydroxycholecalciferols[Title/Abstract]) OR vitamin D3[Title/Abstract]) OR hydroxycholecalciferol[Title/Abstract]) OR dihydroxycholecalciferol[Title/Abstract]) OR vitamin D 3[Title/Abstract]) OR vitamin D[MeSH Terms]) OR cholecalciferol[MeSH Terms]) OR calcitriol[MeSH Terms]). The abovementioned databases were searched with the same keywords as well. Minimal adjustments were made to meet each database's requirements. Spanish equivalents were used rather than English keywords for obtaining the most comprehensive results in the SciELO database.
This search protocol was limited to studies published before 1 Jan 2020. No language, location or any other limitation was applied.
Inclusion and exclusion criteriaTwo authors (AN and HH) performed a separate screening process on all primary results and disagreements were solved by consulting a third author (HV). Considering the limited number of papers, all kind of clinical trials performed on humans were included regardless of patient age or any other variable. Even studies with only abstract were also included if they reported our outcome of interest.
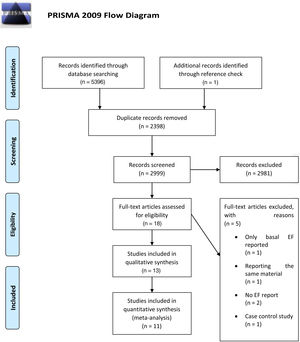
All other study designs (non-clinical trials) and animal studies were excluded, although their bibliography, especially in the case of review articles, was thoroughly explored. Results associated with search strategy are summarized in figure No. 1.
Data extraction and quality assessmentAll papers reporting the outcome of interest of this study (LVEF), were elected for data extraction and quality assessment. Information including name of the first author, publication year, sample size, EF in both control and treatment groups at baseline and end point, patient mean age, duration of treatment with vitamin D and dosage of vitamin D treatment were extracted using EndNote X9 and Microsoft Excel 2013 software. Considering the limited number of studies, we were unable to differentiate HF types based on EF and functional class of HF in separate categories.
For quality assessment, the Cochrane risk of bias tool in randomized trials14 was used. All included papers were appraised by a four level grade15 scoring system (very low, low, moderate, high) for assessing confidence in quality or certainty of a body of evidence. All eligible articles were assessed for quality by two authors independently (AN and HH) and disagreements were solved by consensus and remaining disagreements were resolved by a third author (HV). Whenever required information for quality assessment was not stated clearly within the text, such as in conference and abstract papers, unclear risk of bias was selected.
We sought to maintain a logical balance between originality and uniformity of data during extraction process. Any exceptions are clarified in the data extraction table caption.
Statistical analysisEjection fraction was analysed as a continuous variable with unstandardized mean difference with 95% CI as effect size. Pooled effect was calculated using random effects model and the results are reported as forest plot.
For assessing inter-study heterogeneity, I2, Q statistics and p-value were used and are reported.
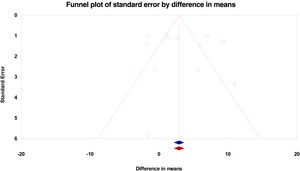
Begg's funnel plot was used for qualitative assessment of publication bias and a two-tailed p value <0.1 for Begg's and Egger's tests were considered statistically significant. Rosenthal's fail-safe number test with a two tailed alpha=0.05 is also reported. Results associated with publication bias are reported as an “imputed and observed funnel plot” rather than p-values and Rosenthal's fail-safe number.
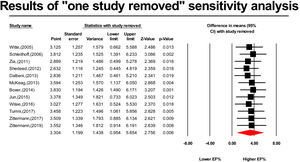
Sensitivity analysis was carried out using the “one study removed” method and the results are reported as a forest plot.
All analyses were performed using comprehensive meta-analysis (version 3) software and in case of any problem or issues, the software handbook and Cochrane handbook were used as a reference.
ResultsSearch resultsAfter a primary search, a total number of 5396 articles were found including PubMed: 582 articles; SCOPUS: 2591 articles; ISI Web of Science: 1263 articles; EMBASE: 944 articles; and SciELO: 16 articles. One article,16 which was not indexed in any of mentioned above databases, was also added after checking the bibliography of a similar systematic review.7 When removing 2398 duplicate articles, 2999 articles were selected for title and abstract screening. Given the search was highly comprehensive and sensitive and with a logical design, 2981 irrelevant articles including animal studies, irrelevant topics or abstract, clearly non-trial studies and lack of reporting our only variable of interest (EF), were individually eliminated in this step. Eighteen papers were selected for the full-text evaluation step, of which five were subsequently removed. Of these five, two articles did not report EF,17,18 one reported baseline EF but not endpoint,19 one article was a case-control study,20 which could not have been excluded by title and abstract alone and one study reported the same material21 of another one which had already been included.22 Among the final 13 articles, one article23 reported results at two different time points and associated results were entered separately in the data extraction table and meta-analysis. Two articles24,25 were not elected for quantitative data synthesis and meta-analysis since they did not report adequate quantitative variables for this goal. The associated qualitative and quality assessment details are summed up in the data extraction table. Finally, 11 studies underwent meta-analysis and the Zittermann et al.23 study is divided into two separate studies. The methodological flow diagram is reported in summary in Figure 1.
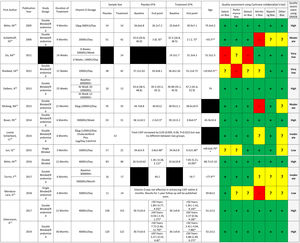
Studies and patients’ characteristicsThirteen clinical trial papers that enrolled a total of 1215 patients were included. The first study was published in 2005 and the most recent in 2019. Mean patients age ranged from 11 months to 78 years. Duration of vitamin D treatment ranged from 12 weeks to three years, with doses starting at 10 μg (400 IU)/day up to 50 000 IU/week. Detailed study and patient information along with results of quality assessment are shown in Figure 2.
Studied patients’ characteristics, and quality assessment. Results are reported in form of mean±SD or mean (lower limit 95% confidence interval, upper limit confidence interval) or median {interquartile range}.
* Cells with one star indicate changes. LVEF: left ventricular ejection fraction.
** Cells with two stars indicate computed measures which are calculated estimates obtained via comprehensive meta-analysis software. Not directly reported in manuscript.
** Shedeed, SA's study with two red stars was performed on infants and mean age is reported as months.
In whole table, black cells indicate missing values.33-38
Each study is followed by associated Cochrane collaboration's tool for quality assessment and GRADE quality assessment chart. In Cochrane collaboration's tool for quality assessment chart, Green color indicates low risk of bias, red color indicates high risk of bias and yellow indicates unclear risk of bias.
Of the 13 articles included for qualitative data synthesis, 11 were entered in meta-analysis and one23 was separated into 2 articles. For Turrini et al.’s study26 we could not obtain the full-text even by contacting the corresponding author, so we used another meta-analysis6 for obtaining our required data. The same happened for Jun et al.’s article16 which was not available on the analyzed databases and was published in a Chinese database. We did our best to enter the maximum number of articles possible for qualitative and quantitative data synthesis even those which were published as conference abstracts.
As illustrated in Figure 3, the results indicate that vitamin D supplementation can significantly (p=0.006) enhance EF in patients with HF by 3.304% (95% CI 0.954, 5.654).
In this study there was very high between study heterogeneity (Q statistic=90.865, I2=87.894 and p=0.0001). In order to reduce the high inter-study heterogeneity, different subgroup analyses (categorical variables) and meta regression models (continuous variables) were applied based on duration of treatment, dosage of vitamin D, age, different HF categories based on EF or baseline EF, but all of them failed to reduce inter-study heterogeneity.
Figure 4 shows the associated funnel plot with both observed and imputed studies. As seen in the funnel plot, no publication bias was detected and Rosenthal's fail-safe number results indicated that 160 missing studies were required to bring the p>0.05. Lack of publication bias is also suggested quantitatively by Egger's two-tailed p=0.68 and Begg's two-tailed p=0.37.
As shown in Figure 5, results from the “one study removed” sensitivity analysis demonstrate that our results are not dependant on any of the included studies and significant vitamin D effect remains when removing each of them.
DiscussionThis study was a comprehensive and focused systematic review and meta-analysis for assessing the efficacy of vitamin D supplements on LVEF in patients with HF.
Systematic reviews are carried out to sum up results of a large number of published papers for final decision making and their difference from narrative and review articles in that systematic reviews have a narrower title. They seek to solve a real clinical challenge27 and use systematic methods to collect relevant data.
As clinicians, we are not yet certain whether patients with HF benefit from vitamin D supplementations. At the moment, HF is mainly defined as a pathological clinical condition with impaired myocardial muscle function and inability of the heart to pump adequate blood levels to meet the metabolic demands of peripheral tissues.28 Reviewing the latest up-to-date literature, several studies and systematic reviews can be found on different HF markers, such as inflammatory markers,29 molecular pathways and signalling.30 Few articles focus on myocardial function indices, of which EF is an important and frequently used one, and few studies are performed in a clinical trial design.
Based on our knowledge, EF is rarely reported in studies and is less pronounced in systematic reviews as the literature trend is mostly focused on molecular and inflammatory indices. Few systematic reviews were performed that reported the effect of vitamin D on EF in patients with HF. Some reported a positive effect7 while others recorded no significant effect.6,11
The number of studies were really limited and even the published systematic reviews are not in agreement on included studies. This seems to be mainly because of different search strategies and databases searches along with lack of focus on a single outcome of interest.
In this study we observed that vitamin D supplements can be significantly effective in enhancing the most important and the most common myocardial function index, EF (p=0.006) with a raw mean difference of 3.304 (95% CI 0.954, 5.654).
The clinical importance of this 3.3% enhancement of EF in patients with HF gains further value when we look back in the literature for the significance of LVEF in patients with HF. In different validated studies and clinical trials, researchers concluded that enhancement in LVEF is directly associated with lower rehospitalization and cardiovascular mortality.8–10
In this systematic review and meta-analysis, we were unable to differentiate the effect of vitamin D in different categories of HF based on EF (such as HF with reduced EF, HF with mid-range EF, HF with preserved EF) since not all studies separated them accurately or reported the results separately.
While performing the statistical analysis, we aimed to at least differentiate HF categories in those studies that reported it, but our results did not indicate any association between category of HF based on EF and efficacy of vitamin D treatment.
Other analyses were also performed in order to find any possible association between dosage of vitamin D or duration of treatment with vitamin D but we could not find any significant association in such analyses.
Across the included studies, mean EF was <50% and given the low rate of adverse effects from vitamin D at the suggested doses or even at higher doses31 and the significance of 3.3% of LVEF in a patients with EF <50%, we believe that vitamin D treatment should be always considered as a treatment for patients with HF.
The most recent systematic review was performed by Wang et al.6 and was published in May 2019. The researchers reported that vitamin D supplementation does not affect LVEF significantly 2.56, (95% CI -2.18, 7.3) which was confirmed in another systematic review performed by Jiang et al.11 in 2016. In this study, the researchers reported that with a mean difference of 4.11 (95% CI -0.91, 9.12), the effect of vitamin D supplements on EF was not significant.
Wang et al.6 published their article on 2019 with a search limited up to October 2017; this is while in another valuable and reliable study conducted by (published in 2018 in BMJ journal and received by journal on November 2017), the researchers claimed a more comprehensive database search which unexpectedly led to a lower number of primary papers. This means that emphasizing on other outcomes rather than EF (as primary outcome) may result in missing papers in which EF is reported as a secondary outcome. In our study, EF was the only outcome of interest, thus we included more studies after a more comprehensive and sensitive search without any limitation.
In Zhao et al.’s study, a significantly positive effect of vitamin D supplements on EF 4.18 (0.36, 7.99) was reported, whereas Wang et al.’s study revealed the opposite. We believe that the controversial results can be due to different primary outcomes in those studies. We are of the opinion that a more focused meta-analysis on EF as a primary outcome, such as our research, can yield better search results.
In this systematic review, we performed a very comprehensive and wide search and all studies were carefully screened by two authors. This accuracy along with help of previous systematic reviews (as we obtained some of our data from them) lead to a total of 11 papers and 12 studies in quantitative data synthesis.
Based on our knowledge, our study is the only systematic review which includes Zittermann et al.’s results after one year and three years23 of follow up. Their study included the largest number of subjects (311 and 242 at one year and three years, respectively) and the results indicate a lack of any total significant effect of vitamin D on EF which contradicts our pooled estimate results.
Our study, like many others, has its own limitations of which we are aware. This study contains a very high inter-study heterogeneity, which can be explained in part by consciously including heterogeneous studies, from patients with a mean age of <1 year to patients <70, a wider range of doses of vitamin D and duration which varied from a few months to three years. Other less significant differences such as gender, genetics and race were also ignored and may also have influenced our results.
As a systematic review and meta-analysis, results of this study were pooled out based on reported outcomes of studies, instead of individual patient data and this means that validated primary researcher and clinical trials are always valuable and required to confirm a scientific hypothesis in clinic.
Another limitation in this paper is that some primary studies included patients with vitamin D deficiency and some enrolled patients with normal levels of vitamin D. On the other hand, many of the primary clinical trials did not even differentiate this important factor and included both patients with and without vitamin D deficiency, which can subsequently reduce the reliability of our results.
Primary trials are not all clear even how EF should be assessed whether it should be performed via echocardiography or cardiac magnetic resonance imaging. Some of them even used a combination and actually measured patients’ EF using different modalities at different time points for the same patient or at the same time point for different patients.
Different categories of HF based on EF such as HF with preserved EF were not discussed and reported. Patient functional class and clinical status were not reported in detail in all studies. In primary trials, the effect of vitamin D was not measured in different patients’ categories according to EF subgroups.
We performed a series of various subgroup analyses and used several meta regression models but unfortunately none of them were problem solving and the heterogeneity remained too high still. We even tried removing the very different studies from all different analyses, such as the Shedeed et al. study,32 but this did not help.
However, returning to the previously mentioned meta analyses, it is notable that they also had a very high heterogeneity and considering all these together, it seems that heterogeneity is somehow inevitable due to the nature of the included studies. The only solution seems to be conducting more high quality clinical trials in this field to enable better meta-analyses in future.
Another reason for performing more primary clinical trials is that among those performed, not all of them were high quality and accurate. These 11 studies which seems to be all available studies, globally does not have an acceptable quality such as clinical trials performed in other fields. We believe that rather than retrospective and descriptive studies, we need additional clinical trials, with enough power to better clarify the role of vitamin D in the treatment of HF.
ConclusionBased on our knowledge this meta-analysis is currently the most up-to-date one with the most comprehensive data about EF. The results indicate that prescribing vitamin D supplements for patients with HF, especially those with EF <50% can enhance myocardial EF.
We conclude that before publication of more primary clinical trials with higher quality and further meta-analysis, it is logical to bear vitamin D supplementation in mind for patients with HF, especially those with vitamin D deficiency.
Funding supportThis study is self-funded and authors did not receive any financial support from any organization for performing this research.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Ana Barradas for her medical writing services and translating the abstract into Portuguese.






![Forest plot indicates a statistically significant effect of vitamin D supplements on ejection fraction in patients with heart failure (p=0.006). Supplementation with vitamin D can increase ejection fraction for 3.304% [95%CI 0.954, 5.654] in heart failure patients. Forest plot indicates a statistically significant effect of vitamin D supplements on ejection fraction in patients with heart failure (p=0.006). Supplementation with vitamin D can increase ejection fraction for 3.304% [95%CI 0.954, 5.654] in heart failure patients.](https://static.elsevier.es/multimedia/21742049/0000004000000006/v1_202107150705/S2174204921001653/v1_202107150705/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)