Beta-blocker doses that have been shown to be effective in randomized clinical trials are not commonly used in daily clinical practice. The aim of this study was to analyze whether there is a prognostic benefit of high rather than low doses of beta-blockers after an acute coronary syndrome (ACS).
MethodsIn this retrospective cohort study, 2092 ACS patients discharged from hospital between June 2013 and January 2016 were classified according to the beta-blocker dose prescribed: high dose (≥50% of the target dose tested in clinical trials) and low dose (<50%). Two groups of 501 matched patients were obtained through propensity score matching according to treatment with high or low doses of beta-blockers. The prognostic impact (mortality) during follow-up of high vs. low dose was analyzed by Cox regression and represented by Kaplan-Meier curves.
ResultsOf the 2092 patients, 80.5% were discharged under beta-blockers, with lower mortality during follow-up (18.6±9.7 months). Of the 1685 patients discharged under beta-blockers, only 31.4% received high doses. There were no differences in mortality during follow-up between patients under high-dose vs. low-dose beta-blockers (HR 0.935, 95% CI 0.628-1.392, p=0.740), and the equivalence between the two doses remained after propensity score matching (HR 1.183, 95% CI 0.715-1.958, p=0.513).
ConclusionNo prognostic benefit was found in terms of mortality for high-dose vs. low-dose beta-blockers after an ACS.
A dose de betabloqueador que demonstrou ser efetiva em ensaios clínicos aleatorizados não é a comummente usada na prática clínica diária. O objetivo deste estudo foi analisar se existe um benefício prognóstico da dose alta versus baixa dose de betabloqueador após uma síndrome coronária aguda (SCA).
MétodosEstudo de coorte retrospetivo, envolveu 2092 doentes com alta após SCA, entre junho de 2013 e janeiro de 2016. Os doentes foram classificados de acordo com a dose de betabloqueador prescrita: alta (≥50% da dose-alvo testada em ensaios clínicos) e baixa (<50%). Foram obtidos dois grupos de 501 doentes emparelhados, por meio de propensity score matching, de acordo com terem sido tratados com alta ou baixa dose de betabloqueadores. O impacto prognóstico (mortalidade) durante o seguimento da dose elevada versus baixa foi analisado pela regressão de Cox e representado pelas curvas de Kaplan-Meier.
ResultadosDos 2092 doentes, 80,5% tiveram alta com betabloqueadores, com uma menor mortalidade durante o seguimento (18,6±9,7 meses). Dos 1685 doentes com betabloqueadores na alta, apenas 31,4% receberam altas doses de betabloqueadores. Não houve diferenças na taxa de mortalidade durante o seguimento entre os doentes com doses altas versus doses baixas de betabloqueadores (HR 0,935, IC 95% 0,628-1,392, p=0,740), manteve-se a equidade entre as duas doses após o propensity score matching (HR 1,183, IC 95% 0,715-1,958, p=0,513).
ConclusãoNão foi encontrado qualquer benefício prognóstico em termos de mortalidade entre doses elevadas e doses baixas de betabloqueadores após uma SCA.
Therapy with beta-blockers is recommended for patients after an acute coronary syndrome, but the most commonly prescribed doses are less than half those evaluated in the randomized clinical trials that demonstrated efficacy, and optimum doses have not been validated. The data of our study do not demonstrate increased survival in patients treated with beta-blocker doses approximating those used in previous randomized clinical trials compared with lower doses. These findings provide the rationale to investigate the appropriate beta-blocker dosage after acute coronary syndrome to derive optimal benefit from this therapy.
IntroductionBeta-blocker treatment is part of the therapeutic arsenal commonly used after an acute coronary syndrome (ACS). The prognostic benefit of these drugs after an ACS was established by studies carried out in the era prior to percutaneous revascularization1,2 as well as by studies on patients with left ventricular systolic dysfunction.3 However, based on data from multiple contemporary non-randomized studies,4–6 clinical practice guidelines consider it reasonable to extrapolate their prognostic benefit to the full spectrum of ACS patients, unless there is an established contraindication.7–10
While the recommendation for beta-blockers after an ACS is based on solid scientific evidence, the type and dosage are not consensual. Clinical trials used high doses of beta-blockers to establish the efficacy of this medication,1,2,11 however such high doses are often not tolerated and are not used in daily clinical practice.12 Based on available clinical trials and extrapolation of existing evidence in the field of heart failure,13 it seems reasonable to prescribe the beta-blocker doses used in these studies, or at least titrate to the highest dose tolerated by the patient. However, there is little evidence on this question, and the available data are controversial.14,15
This study aims to analyze whether there are prognostic differences between high and low doses of beta-blockers in terms of long-term survival after an ACS.
MethodsStudy populationThis retrospective cohort study included all consecutive patients admitted to the cardiology department of the Álvaro Cunqueiro University Hospital of Vigo between June 2013 and January 2016 with a diagnosis of ACS and who underwent coronary angiography (n=2702). Patients with no evidence of angiographically significant coronary lesions (n=432) were excluded from the original registry, as were those who died during hospital stay (n=102). Follow-up data were obtained in 96.6% of these 2168 patients (74 patients without follow-up data), so the study cohort comprised 2092 patients. Demographic, clinical and angiographic data, as well as information on treatment and follow-up, were collected and reviewed prospectively by cardiologists of this department. The study was carried out in accordance with the principles of the Declaration of Helsinki.
Beta-blocker dosesPatients were classified according to the beta-blocker dose prescribed at discharge. For the purposes of the study, the optimal daily doses based on clinical trials (target dose) were 10 mg for bisoprolol, 50 mg for carvedilol, 200 mg for metoprolol, and 10 mg for nebivolol. A high dose was defined as ≥50% of the daily target dose.
Aim of the study and follow-upPatients were classified according to whether they were treated with high or low beta-blocker doses. Treatment was at the discretion of the attending cardiologist. The study's primary endpoint was the effect of high vs. low dose of beta-blockers on total mortality at follow-up (18.6±9.7 months). After discharge, patients were followed in an ischemic heart disease outpatient clinic and in primary care. Structured follow-up was carried out through electronic history. All medical care and hospital records were reviewed and telephone contact was used in certain cases.
Statistical analysisQuantitative variables were expressed as means and standard deviation. The Student's t test was used for comparison between the two groups. Qualitative variables were expressed as percentages and compared using the chi-square test. Given the non-randomized nature of the study and the many factors that could influence the use of high-dose beta-blocker therapy, in order to reduce the bias involved in studying the effect of an observational study, a propensity score analysis was conducted, in which the characteristics of the two groups (high and low beta-blocker dose) were matched. A greedy protocol of 1:1 was used without replacement, accepting as optimal a standard deviation of 0.1. This analysis was performed using binary logistic regression, in which the dependent variable was treatment with high dose of beta-blockers (yes/no) and the explanatory variables were age, female gender, diabetes, peripheral arterial disease, chronic obstructive pulmonary disease (COPD), previous heart failure, history of cancer, atrial fibrillation, bundle branch block, serum creatinine, current admission for ST-segment elevation myocardial infarction (STEMI), peak troponin I, significant left main coronary artery (>50%) or proximal left anterior descending coronary artery (>70%) stenosis, percutaneous coronary intervention (PCI), coronary artery bypass graft surgery and complete revascularization. Using propensity score matching, two groups were obtained of 501 patients matched in their propensity to receive high-dose beta-blockers. In the matched cohort, event-free survival was analyzed using the Kaplan-Meier method, with the log-rank test for comparison between groups. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for high-dose vs. low-dose beta-blockers in the total cohort and in specific population subgroups (ACS type, left ventricular ejection fraction ≤40%, and complete revascularization). Statistical analyses were performed using SPSS version 21.0 for Windows and R package. A value of p<0.05 was considered statistically significant.
ResultsOf the 2702 discharged ACS patients, 1685 were treated with beta-blockers (80.5%). Table 1 shows the characteristics of patients stratified by use of beta-blockers. The most commonly used beta-blocker was bisoprolol (n=1435, 85.2%), followed by carvedilol (n=210, 12.5%), atenolol (n=20, 1.2%), nebivolol (n=16, 0.9%) and metoprolol (n=4, 0.2%). Of the patients discharged with beta-blockers, 190 were treated with <25% of the optimal target dose (11.3%), 967 with doses between 25 and 49.9% of target dose (57.4%), 453 with doses between 50 and 74.9% (26.9%), and only 75 with doses ≥75% of target dose (4.5%). Thus, only 31.4% of patients (n=528) were discharged with beta-blocker doses ≥50% of the optimal target dose. Patients who were prescribed high doses were younger, with a higher rate of hypertension and previous myocardial infarction (MI) and a lower rate of COPD, more frequently with ventricular dysfunction and multivessel disease, and a lower rate of complete revascularization (Table 2).
Baseline characteristics of patients stratified by use of beta-blockers.
| Variable | Beta-blocker (n=1685; 80.5%) | No beta-blocker (n=407; 19.5%) | p |
|---|---|---|---|
| Age, years | 64.6±12.8 | 68.9±13.2 | <0.001 |
| Female, % | 21.5 | 27.0 | 0.018 |
| Diabetes, % | 26.4 | 29.7 | 0.176 |
| Hypertension, % | 68.3 | 59.3 | 0.001 |
| Dyslipidemia, % | 66.5 | 66.8 | 0.890 |
| COPD, % | 7.7 | 20.6 | <0.001 |
| PAD, % | 8.3 | 12.0 | 0.018 |
| Previous MI, % | 14.4 | 13.8 | 0.732 |
| Cancer, % | 9.6 | 15.0 | 0.001 |
| STEMI | 49.1 | 32.2 | <0.001 |
| Killip class ≥II | 14.1 | 12.3 | 0.349 |
| LVEF ≤40% | 17.3 | 11.3 | 0.002 |
| AF, % | 9.7 | 12.3 | 0.118 |
| Hemoglobin, g/dl | 14.1±2.0 | 13.9±1.8 | 0.264 |
| sCr, mg/dl | 1.0±0.8 | 1.0±0.6 | 0.874 |
| Multivessel CAD, % | 57.9 | 52.1 | 0.035 |
| LMCA, % | 8.9 | 6.9 | 0.189 |
| PCI, % | 83.0 | 82.6 | 0.821 |
| DES, % | 65.0 | 63.4 | 0.531 |
| Complete revascularization, % | 51.2 | 49.6 | 0.578 |
| In-hospital reinfarction, % | 1.8 | 2.2 | 0.564 |
| In-hospital HF, % | 6.4 | 5.8 | 0.627 |
| In-hospital sustained VA, % | 3.3 | 2.2 | 0.268 |
| DAT, % | 88.2 | 85.7 | 0.167 |
| ACEI or ARB, % | 66.8 | 61.0 | 0.027 |
| Statin, % | 96.0 | 91.3 | <0.001 |
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DAT: dual antiplatelet therapy; DES: drug-eluting stent; HF: heart failure; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; sCR: serum creatinine; STEMI: ST-elevation myocardial infarction; VA: ventricular arrhythmias.
Baseline characteristics of patients stratified by use of high vs. low beta-blocker dose.
| Variable | High dose (n=528; 31.3%) | Low dose (n=1157; 68.7%) | p |
|---|---|---|---|
| Age, years | 63.5±12.1 | 65.0±13.1 | 0.021 |
| Female, % | 19.5 | 22.5 | 0.170 |
| Diabetes, % | 27.8 | 25.8 | 0.368 |
| Hypertension, % | 62.9 | 57.7 | 0.046 |
| Dyslipidemia, % | 69.3 | 65.2 | 0.094 |
| COPD, % | 5.3 | 8.8 | 0.012 |
| PAD, % | 8.0 | 8.5 | 0.722 |
| Previous MI, % | 18.2 | 12.7 | 0.003 |
| Cancer, % | 7.8 | 10.4 | 0.091 |
| STEMI | 44.7 | 51.1 | 0.012 |
| Killip class ≥II | 11.4 | 15.3 | 0.031 |
| LVEF ≤40% | 21.8 | 15.9 | 0.003 |
| AF, % | 8.3 | 10.3 | 0.209 |
| Hemoglobin, g/dl | 14.2±2.0 | 14.0±2.0 | 0.054 |
| sCr, mg/dl | 1.0±0.7 | 1.0±0.8 | 0.873 |
| Multivessel CAD, % | 62.1 | 55.9 | 0.017 |
| LMCA, % | 8.7 | 9.0 | 0.853 |
| PCI, % | 81.8 | 83.6 | 0.372 |
| DES, % | 65.5 | 64.8 | 0.778 |
| Complete revascularization, % | 45.0 | 54.0 | 0.001 |
| In-hospital reinfarction, % | 0.8 | 2.2 | 0.032 |
| In-hospital HF, % | 4.5 | 6.3 | 0.149 |
| In-hospital sustained VA, % | 2.8 | 3.5 | 0.509 |
| DAT, % | 87.3 | 88.7 | 0.419 |
| ACEI or ARB, % | 70.7 | 65.0 | 0.021 |
| Statin, % | 96.6 | 95.7 | 0.381 |
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DAT: dual antiplatelet therapy; DES: drug-eluting stent; HF: heart failure; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; sCR: serum creatinine; STEMI: ST-elevation myocardial infarction; VA: ventricular arrhythmias.
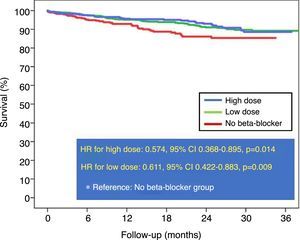
During follow-up (18.6±9.7 months), 114 patients died (6.8%). Patients who received beta-blockers had lower mortality than those who did not (unadjusted HR: 0.599, 95% CI 0.423-0.848, p=0.004). Compared with patients who were not prescribed beta-blockers, unadjusted HR was similar for high doses (HR: 0.574, 95% CI 0.368-0.895, p=0.014) and low doses (HR: 0.611, 95% CI 0.422-0.883, p=0.009), with no difference between the two doses (Figure 1).
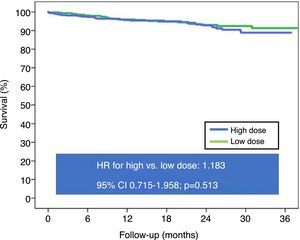
After propensity score matching (Table 3), there were no statistically significant differences in post-discharge mortality between the two groups (Figure 2). Analysis by subgroups (Table 4) also did not reveal a prognostic benefit of high beta-blocker doses. Even in patients with STEMI, high-dose beta-blockers were associated with higher mortality than low-dose beta-blockers (HR: 2.998, 95% CI 1.190-7.557, p=0.020).
Characteristics of high-dose vs. low-dose beta-blocker groups after propensity score matching.
| Variables | High-dose BB (n=501) | Low-dose BB (n=501) | p |
|---|---|---|---|
| Age, years | 63.4±12.1 | 63.8±13.3 | 0.659 |
| Female, % | 19.6 | 20.6 | 0.693 |
| Diabetes, % | 26.5 | 27.7 | 0.670 |
| Hypertension, % | 61.5 | 63.3 | 0.557 |
| Dyslipidemia, % | 68.3 | 68.7 | 0.633 |
| COPD, % | 5.4 | 6.0 | 0.682 |
| PAD, % | 8.2 | 8.0 | 0.908 |
| Previous MI, % | 16.2 | 19.0 | 0.245 |
| Previous HF, % | 2.2 | 3.8 | 0.138 |
| Cancer, % | 8.0 | 6.6 | 0.395 |
| STEMI | 46.1 | 47.1 | 0.835 |
| Killip class ≥II | 11.4 | 13.2 | 0.386 |
| LVEF ≤40% | 19.0 | 19.2 | 0.936 |
| AF, % | 8.6 | 9.6 | 0.583 |
| Hemoglobin, g/dl | 14.3±1.8 | 14.1±1.8 | 0.262 |
| sCr, mg/dl | 1.0±0.8 | 1.0±0.9 | 0.577 |
| Multivessel CAD, % | 60.9 | 61.3 | 0.897 |
| LMCA, % | 8.0 | 10.2 | 0.227 |
| PCI, % | 82.6 | 83.0 | 0.867 |
| DES, % | 65.7 | 65.1 | 0.842 |
| Complete revascularization, % | 41.5 | 42.1 | 0.848 |
| In-hospital reinfarction, % | 0.8 | 1.4 | 0.363 |
| In-hospital HF, % | 4.8 | 5.8 | 0.480 |
| In-hospital sustained VA, % | 2.8 | 2.6 | 0.845 |
| DAT, % | 88.0 | 87.2 | 0.701 |
| ACEI or ARB, % | 68.9 | 72.7 | 0.187 |
| Statin, % | 96.4 | 96.0 | 0.741 |
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DAT: dual antiplatelet therapy; DES: drug-eluting stent; HF: heart failure; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; sCR: serum creatinine; STEMI: ST-elevation myocardial infarction; VA: ventricular arrhythmias.
Subgroup analysis comparing high and low beta-blocker doses after propensity score matching.
| Subgroup | HR | 95% CI | p | |
|---|---|---|---|---|
| Age >75 years | Yes | 1.153 | 0.588-2.259 | 0.679 |
| No | 1.393 | 0.646-3.002 | 0.397 | |
| STEMI | Yes | 2.998 | 1.190-7.557 | 0.020 |
| No | 0.691 | 0.358-1.331 | 0.269 | |
| LVEF ≤40% | Yes | 1.103 | 0.503-2.420 | 0.806 |
| No | 1.253 | 0.649-2.419 | 0.501 | |
| Complete revascularization | Yes | 2.546 | 0.799-8.119 | 0.114 |
| No | 0.939 | 0.530-1.663 | 0.828 | |
CI: confidence interval; HR: hazard ratio; LVEF: left ventricular ejection fraction; STEMI: ST-segment elevation myocardial infarction.
The present study was designed to assess whether there is a prognostic benefit in terms of survival of high doses (≥50% of the target dose previously used in randomized controlled trials) vs. low doses of beta-blockers in patients discharged after an ACS. The results were contrary to the initial hypothesis, demonstrating an absence of reduction in mortality with high compared to low doses of beta-blockers. Although there were differences in baseline characteristics, mode of presentation of ACS and angiographic characteristics that could have influenced the prescription of high vs. low beta-blocker doses, the absence of a prognostic benefit of high doses remained after adjustment by propensity score matching.
Clinical trials of beta-blockers have used high target doses. However, this is not the usual prescription pattern in daily clinical practice.16 In our study, only 31.3% of patients received ≥50% of the target dose proposed in these studies. This is consistent with data from the Outcomes of Beta-blocker Therapy After Myocardial Infarction (OBTAIN) registry and the Intermountain Heart Collaborative Study (IHCS), in both of which fewer than 20% of patients received ≥50% of the target dose.14,15 Metoprolol was the most used beta-blocker in both studies, as opposed to bisoprolol in our study. It should be noted that clinical trials with beta-blockers in MI have not provided information on dose response, focusing only on the target dose, but this target dose is rarely tolerated in daily clinical practice with real-world patients.17,18 Hence the importance of the question posed in our work: are there prognostic differences between low-dose and high-dose beta-blockers?
Our study's findings show equivalence in terms of mortality, without finding a benefit of high vs. low beta-blocker doses. Although low doses were associated with advanced age, more COPD, and less use of angiotensin-converting enzyme inhibitors (ACEIs), after the analysis was adjusted by propensity score matching, no prognostic differences were found regarding the use of high doses of beta-blockers. Although these results are somewhat surprising, and contrary to the initial hypothesis, they are consistent with the results of recent studies by Goldberger et al.14 and Allen et al.15 It should be noted that as early as 1998, Barron et al. found in 1165 patients who had survived MI that those receiving low-dose beta-blockers (<50% of the optimal dose tested in clinical trials) had a greater reduction in mortality (HR 0.33 for low dose vs. no beta-blockers, p=0.009; HR 0.82 for high dose vs. no beta-blockers, p=0.510).12 More recently (2015), in the OBTAIN registry, patients treated with ≤25% of the target dose appeared to show better survival than those receiving ≥50%,14 and data from the ICHS registry, also contemporary (2016), were consistent with our findings, with no differences between the different beta-blocker doses.15
The marked prognostic benefit of beta-blockers in survival after an ACS, demonstrated in the 1980s and 1990s,19 based on the reduction of ischemia, reinfarction and ventricular tachyarrhythmias, has been mitigated in the current era by generalization of PCI and the extension of optimal medical therapy with dual antiplatelet therapy, statins and ACEIs to the majority of patients. This reduction in their prognostic impact may add to the potential negative prognostic impact of bradyarrhythmias, as demonstrated in the CHARISMA study, in which up to 17% of patients treated with beta-blockers at high doses after MI with LVEF <40% had sinus bradycardia or high-grade atrioventricular block.20 Furthermore, the adverse events associated with beta-blockers – depression, fatigue and sexual dysfunction – should also be taken into consideration.18 This has led in recent years to questioning of the clinical utility of beta-blockers after an ACS, with conflicting results generating some degree of confusion, although current evidence seems to favor beta-blocker therapy, even in patients with LVEF >40%.4–6 What is not clear is the dose to be used. There may not be a single optimal dose of beta-blockers for all patients; dosage should be individually adjusted, as some patients will benefit from low doses and others from high doses. Some studies suggest that beta-adrenergic receptor polymorphisms may play an important role in patients with ACS and heart failure.21 On the other hand, other studies have suggested that the reduction in post-MI mortality with beta-blockers is due to the degree of reduction in heart rate rather than to the dose or type of the beta-blocker itself.22,23 Thus, there are various individual factors that could affect the prognostic benefit of beta-blocker dose.
There are several limitations that should be considered when interpreting the results of our study. First, it was not randomized, and suffers from the usual limitations and biases inherent to retrospective analyses. It is worth noting that it was based on the principle of intention to treat, since it is not possible to assess adherence to or changes in treatment during follow-up. It should also be noted in this regard that, according to previous studies, only a minority of patients undergo changes in beta-blocker therapy in the first three years after MI. Another potential limitation is the use of different types of beta-blockers, although most patients were treated with bisoprolol. Moreover, although analysis by propensity score is more robust than classic regression, it suffers from certain weaknesses compared to a randomized clinical trial, such as the inability to correct for unmeasured confounding factors. Also, given the retrospective nature of the study, it was not possible to analyze other endpoints that might have been of interest, such as control of anginal symptoms, blood pressure, heart rate or arrhythmic events. Despite these limitations, our results showing the lack of benefit of high vs. low doses of beta-blockers are consistent with the two recently published studies discussed above. Thus, the combined findings of all these papers form a consistent basis for guiding future clinical trials that may have a clinical impact on daily medical practice.
ConclusionsNo prognostic benefit was found for high doses of beta-blockers (≥50% of the maximum recommended dose) compared to low doses. Given this finding, we emphasize the need to re-evaluate the role of beta-blocker dose in the current era of coronary revascularization and optimal medical treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all the health and non-health personnel of the cardiology department of the University Hospital Álvaro Cunqueiro de Vigo, for their outstanding work and their involvement in the search for excellence in clinical care.