This study aims to assess the thickness of epicardial fat tissue (EFT), a sign of cardiovascular risk, using echocardiography in patients with severe periodontitis.
MethodsThirty-three patients with stage III or IV periodontitis and 33 healthy participants were enrolled into the study. Epicardial fat tissue thickness was measured perpendicularly via echocardiography of the free wall of the right ventricular at end-diastole in three cardiac cycles. Body mass index (BMI) was calculated by dividing weight in kilograms by the height in meters squared. EFT to BMI ratio (EFT/BMI) was measured by dividing EFT by the BMI.
ResultsThere was no significant difference between study patients and the control group as regards to the frequency of diabetes, hypertension, smoking, and hyperlipidemia. The EFT and EFT/ BMI ratio were significantly different in the control and periodontitis groups (0.51±0.17 vs. 0.77±0.16, respectively; p ≤0.001) (0.021±0.008 vs. 0.030±0.006, respectively; p≤0.001). Pearson's correlation coefficient demonstrated a significant relationship between EFT and the clinical parameters of periodontitis (p<0.001)
ConclusionsEFT thickness measured by echocardiography appears to be associated with severe periodontitis and may thus be an indirect sign of cardiovascular disease in periodontitis patients.
Este estudo visa a avaliar a espessura do tecido adiposo epicárdico (TAE), um sinal de risco cardiovascular, utilizando ecocardiografia em doentes com periodontite grave.
MétodosForam incluídos no estudo 33 doentes com periodontite de fase III ou IV e 33 participantes saudáveis. A espessura do tecido adiposo epicárdico foi medida por ecocardiografia da parede livre do ventrículo direito no fiml do diastol. O índice de Massa Corporal (IMC) é calculado dividindo o peso em quilogramas pelo quadrado de altura em metros. O rácio TAE/IMC foi medido dividindo TAE pelo valor IMC.
ResultadosNão houve diferença significativa entre os pacientes do estudo e o grupo controle em relação às frequências de diabetes mellitus, hipertensão, tabagismo e hiperlipidemia. TAE e TAE/IMC rácio foram significativamente diferentes entre o controle e a periodontite grupos (de 0,51±0,17 versus 0,77±0,16, respetivamente; p≤0,001) (0,021±0,008 versus 0,030±0,006, respetivamente; p≤0,001).
ConclusõesA espessura da TAE medida por ecocardiografia parece estar associada a periodontite grave e pode, portanto, ser um sinal indirecto de doenças cardiovasculares em doentes com periodontite.
Periodontitis is a chronic multifactorial inflammatory disease induced by dysbiotic plaque biofilms and characterized by progressive destruction of the dental supporting tissues.1 The harmful effects of periodontitis are not only limited to the oral cavity but have an impact on general health. Periodontitis may cause bacteremia, endotoxemia, and systemic low-grade inflammation which has been implicated as a potential risk factor for the onset and development of systemic diseases such as cardiovascular diseases (CVDs), rheumatoid arthritis, adverse pregnancy outcomes, respiratory diseases, and diabetes mellitus.2–5 Recent studies have shown a strong relationship between periodontitis and systemic diseases including cardiovascular diseases and diabetes.6–8
Epicardial fat tissue (EFT), a visceral fat tissue of the heart, has mechanical, thermogenic, metabolic and endocrine/paracrine functions.9,10 The functional complexity of EFT remains unclear. EFT has been shown to have effects on cardiac morphology and function, contributing to the preservation of contractility and repolarization of the heart in physiological conditions.11 Besides, it accounts for a major source of a series of proatherogenic and proinflammatory hormones and cytokines such as tumor necrosis factor-α, interleukin-1, interleukin-6, nerve growth factor, leptin and adiponectin.12 Such adipokines and cytokines may affect the myocardium and coronary arteries through paracrine and/or vasocrine mechanism, contributing to the development of atrial fibrillation and coronary artery disease.13 The mechanisms regulating the balance between protective and harmful effects of epicardial adipose tissue are yet to be enlightened.
More recent studies have suggested that EFT may be associated with systemic inflammation. In a recent study of Ertem et al., EFT has been shown to increase in patients with Lichen planus, a mucocutaneous inflammatory disease, compared to control subjects.14
Epicardial adipose tissue might contribute to the development of atherosclerosis through an inflammatory mediated process.9,15 Some studies have reported a strong correlation between atherosclerosis and EFT thickness.16,17 Likewise, such correlation has been demonstrated with obesity, metabolic syndrome, hypertension, and diabetes as well.13,18,19 Although the role of periodontitis as an independent risk factor for atherosclerotic CVDs is established, there is no study evaluating a possible relationship between periodontitis and EFT. This study aimed to make an echocardiographic assessment of EFT thickness as a sign of cardiovascular risk in patients with severe periodontitis.
MethodsStudy populationThe present cross-sectional study was conducted at Bolu Abant Izzet Baysal University Hospital between July 2018 and December 2018. Approval for the study protocol has been granted by the local Ethics Committee. Following the exclusion procedure, we included thirty-three patients having severe periodontitis and thirty-three healthy individuals in our study.
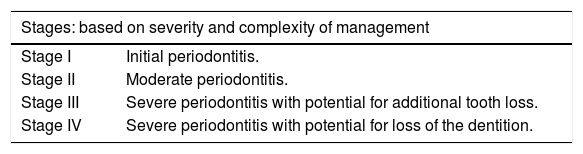
The participants were either classified as having stage III or IV periodontitis or periodontally healthy subjects, based on the criteria suggested by the 2017 International Workshop for Classification of Periodontal Diseases and Conditions (Table 1).1
The 2017 Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.
| Stages: based on severity and complexity of management | |
|---|---|
| Stage I | Initial periodontitis. |
| Stage II | Moderate periodontitis. |
| Stage III | Severe periodontitis with potential for additional tooth loss. |
| Stage IV | Severe periodontitis with potential for loss of the dentition. |
Patients meeting the following criteria were enrolled into the study: no recent minor infection (e.g., flu or sore throat) that requires antibiotic therapy in the past 4-8 weeks, probing depth (PD) ≥6 mm, clinical attachment level (CAL) ≥5 mm, and radiographic bone loss extending to mid-third of root and beyond. The inclusion criteria for periodontally healthy subjects were as follows: no site with clinical attachment loss, no site with probing depth (PD) >3 mm, and no bone loss on radiographs.
We also excluded those having a history of atherosclerotic cardiovascular disease, heart failure, structural heart disease, chronic lung disease, liver or renal failure, thyroid disorders, diabetes, hypertension, hyperlipidemia, smoking, malignancies, electrolyte imbalances or any other systemic disease.
Clinical examinationAll clinical parameters were evaluated by a single experienced periodontist (G.U) and a calibration exercise was performed to obtain acceptable interexaminer reproducibility. Periodontal examinations were performed with a Williams probe (Hu-Friedy, Chicago, IL, USA). The clinical parameters of PD, plaque index (PI) and clinical attachment level (CAL) were measured for every tooth present in the oral cavity. The measurements were performed at six sites (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual) and the results were recorded in approximation to the nearest whole millimeter. The distance from the bottom of the pocket to the cementoenamel junction was defined as CAL and this distance between these two points was measured and recorded. The mean PD and the mean CAL values were calculated by dividing the total score of all teeth by the total number of teeth examined during the study. The periodontal probe was carefully and gently introduced into the gingival sulcus to calculate the percentage of BOP, even one site with BOP was recorded as (+) for each tooth.
EchocardiographyWe conducted all echocardiographic examinations using a 4-Mhz transducer of Vivid S6 (GE Vingmed, N-3191 Horten-Norway). Echocardiological assessments were performed by a cardiologist blinded to the study. One-lead electrocardiography (ECG) was recorded continuously, and the average of three consecutive cycles was calculated for each parameter measured during the echocardiographic examination. Two-dimensional and pulsed Doppler measurements were employed based on the criteria of the American Society of Echocardiography.20
The following parameters were recorded by echocardiography: left ventricular end-diastolic diameter (LVEDD, mm), left atrium diameter (LAD), left ventricular ejection fraction (LVEF, %), and EFT. The LVEF was predicted using Simpson's rule. Lacobelli's method was used to measure the EFT.21
Epicardial fat was detected in the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium, and calculated at the end-diastole in three cardiac cycles perpendicularly on the free wall of the right ventricle. We leveraged the mean of the maximum values measured at any site. Body Mass Index (BMI) was calculated dividing the body weight in kilograms by the square of the height in meters. The ratio of EFT to BMI (EFT/BMI) was calculated dividing EFT by BMI. To increase confidence in the results, EFT measurement was performed at two different times and the percentage of the R-R interval with the least amount of motion was used.
Statistical analysisThe minumum required sample size has been calculated using the G*Power software version 3.1.9.4 (Heinrich Heine University, Dusseldorf, Germany). A power analysis was performed based on a previous study.22 It is estimated that a sample size of 30 participants per- group would have a power of 80% to detect a statistically significant difference between two groups as regard the EFT thickness (alpha level=0.05, effect size=0.75).
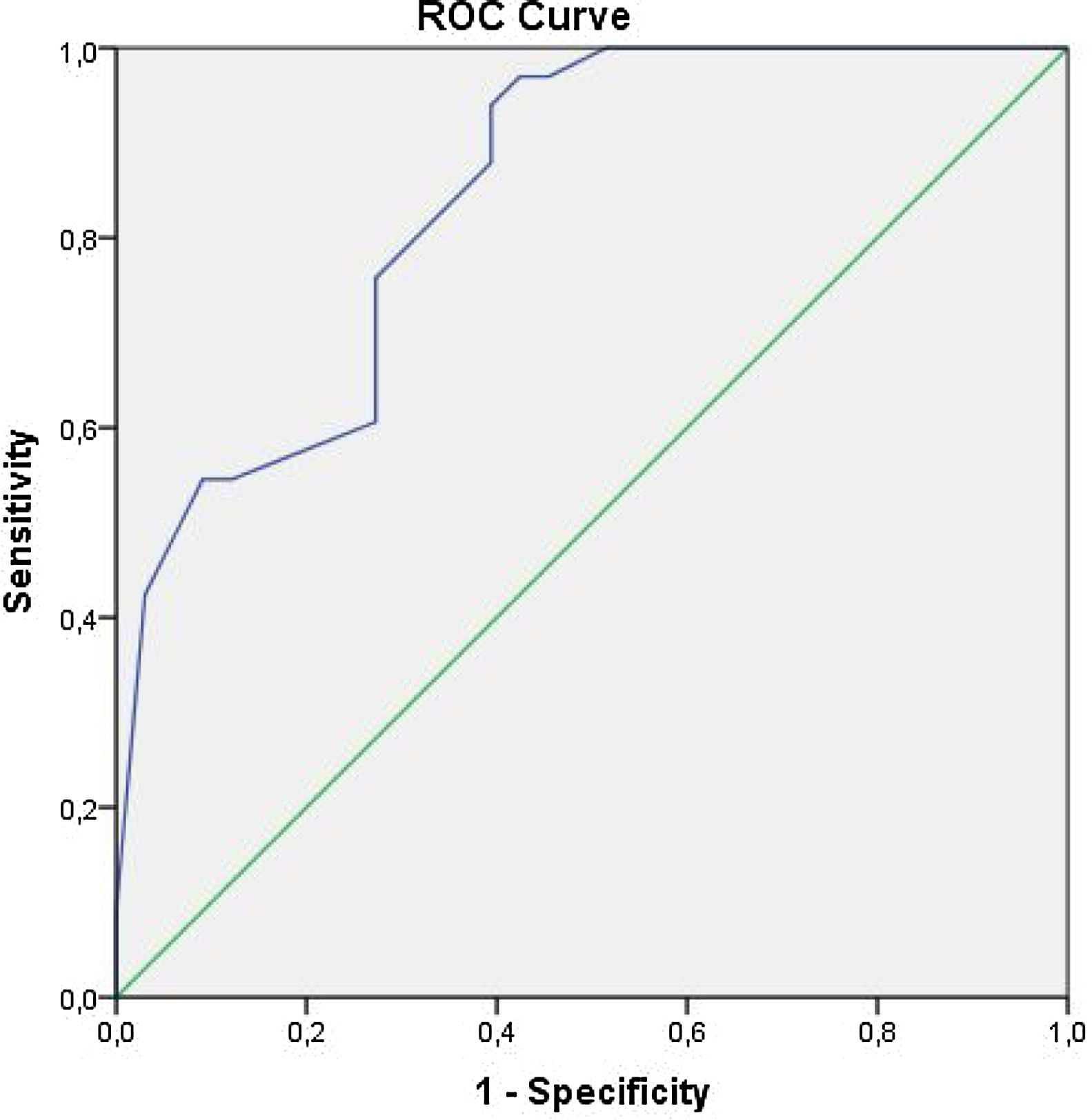
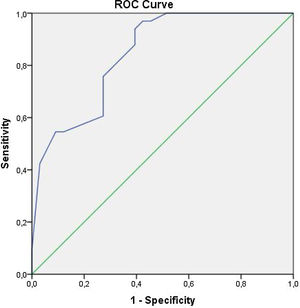
We carried out analyses using SPSS 16.0 Statistical Package Software for Windows Operating System (SPSS Inc, Chicago, Illinois, USA). Quantitative and qualitative variables were expressed as mean±standard deviation (SD), and numbers and percentages, respectively. To assess the differences between these groups, we used Student t-test for normally distributed variables, Mann-Whitney's U-test for variables without normal distribution, and Chi-square test for qualitative variables. A receiver operating curve (ROC) analysis was used to find the sensitivity and specificity of EFT to predict the presence of periodontitis. Pearson test was used for correlation analysis. All results at p<0.05 were considered statistically significant.
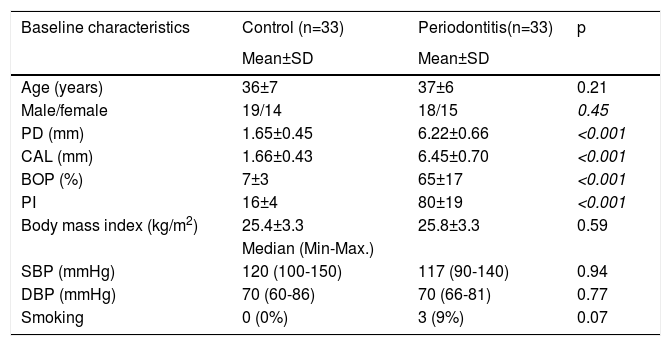
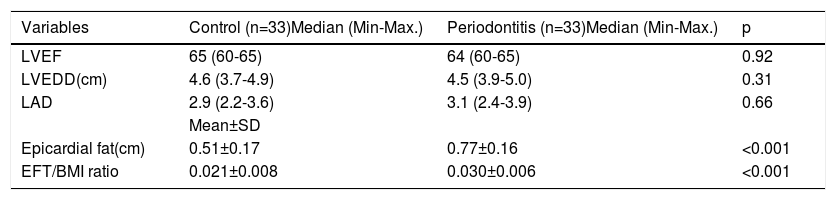
ResultsThe study population included a total of 66 patients of whom 33 were assigned to the control group and 33 to the periodontitis group. The mean ages were 37±6 and 36±7 years for the periodontitis group and the healthy group, respectively. There were no significant differences in age and gender between the study groups (p>0.05). PD, CAL, BOP, and PI were found to be statistically different between the groups (p<0.001) (Table 2). There was no significant difference between study patients and the control group as regards the frequencies of diabetes mellitus, hypertension, smoking, and hyperlipidemia (Table 2). Echocardiographic parameters including LVEF, LVEDD, and LAD were similar between study groups. However, the EFT and EFT/BMI ratio were significantly different between the control and periodontitis groups (0.51±0.17 v.s 0.77±0.16, respectively; p≤0.001) (0.021±0.008 v.s 0.030±0.006, respectively; p≤0.001) (Table 3).
Baseline characteristics of the study population.
| Baseline characteristics | Control (n=33) | Periodontitis(n=33) | p |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (years) | 36±7 | 37±6 | 0.21 |
| Male/female | 19/14 | 18/15 | 0.45 |
| PD (mm) | 1.65±0.45 | 6.22±0.66 | <0.001 |
| CAL (mm) | 1.66±0.43 | 6.45±0.70 | <0.001 |
| BOP (%) | 7±3 | 65±17 | <0.001 |
| PI | 16±4 | 80±19 | <0.001 |
| Body mass index (kg/m2) | 25.4±3.3 | 25.8±3.3 | 0.59 |
| Median (Min-Max.) | |||
| SBP (mmHg) | 120 (100-150) | 117 (90-140) | 0.94 |
| DBP (mmHg) | 70 (60-86) | 70 (66-81) | 0.77 |
| Smoking | 0 (0%) | 3 (9%) | 0.07 |
PD: Probing Depth; CAL: Clinical attachment level; BOP: Bleeding on probing; PI: Plaque index; SD: Standard Deviation; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Echocardiographic parameters data of the study cohorts.
| Variables | Control (n=33)Median (Min-Max.) | Periodontitis (n=33)Median (Min-Max.) | p |
|---|---|---|---|
| LVEF | 65 (60-65) | 64 (60-65) | 0.92 |
| LVEDD(cm) | 4.6 (3.7-4.9) | 4.5 (3.9-5.0) | 0.31 |
| LAD | 2.9 (2.2-3.6) | 3.1 (2.4-3.9) | 0.66 |
| Mean±SD | |||
| Epicardial fat(cm) | 0.51±0.17 | 0.77±0.16 | <0.001 |
| EFT/BMI ratio | 0.021±0.008 | 0.030±0.006 | <0.001 |
LVEF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; LAD: left atrium diameter; EFT: epicardial fat thickness; BMI: body mass index.
ROC analysis has revealed that any EFT value greater than 0.610 cm has a sensitivity of 76% and specificity of 73% in predicting periodontitis groups (Figure 1). Pearson's correlation analysis demonstrated a significant relationship between EFT and clinical parameters of periodontitis (p<0.001) (Table 4).
This study aimed to compare the EFT thickness parameters between patients with severe periodontitis and periodontally healthy subjects to reveal its role both in the CVDs and periodontal diseases by echocardiography. According to our results, EFT thickness was found significantly higher in the periodontitis group compared to the periodontally healthy group. One possible underlying mechanism may be persistent inflammation. EFT thickness is correlated with several circulating proatherogenic and proinflammatory adipokines such as TNF-α, MCP-1, and IL-6 which also play a significant role in periodontal diseases.
Although cardiovascular magnetic resonance (MR) is the gold standard for imaging EFT, it is expensive and difficult to apply.23 However, Iacobellis et al. showed in 2003 that echocardiographic measurements of EFT correlated well with epicardial fat measured by MRI,24 leading us to prefer echocardiography as a cheap, harmless and easy to perform the method.
Previous studies have shown the relationship between inflammatory diseases and EFT. Bacaksız et al.25 demonstrated that EFT significantly increased in patients with psoriasis vulgaris, an inflammatory disease. Likewise, Al-Talabany et al.26 showed that IL-6, an inflammation mediator, had a strong association with EFT in patients with cardiovascular disease and type 2 diabetes. Moreover, Ozdil et al.27 reported that the EFT values of patients with inflammatory bowel diseases were higher than those of the healthy control group. In our study, EFT measurements were also significantly higher in patients with periodontitis as well as with other inflammatory diseases.
The systematic inflammatory response results in endothelial dysfunction in periodontitis, thus contributing to cardiovascular diseases.28 EFT has also been identified as an important source of pro-inflammatory mediators which deteriorate the endothelial dysfunction, eventually leading to coronary artery disease.29
Positive and strong correlations between atherosclerosis and epicardial fat tissue thickness have been shown in recent studies.30,31 Xu et al.32 reported in a recent meta-analysis of 2.872 patients that both EFT and EFT volumes significantly increased in patients with coronary artery disease (CAD) compared to those in the healthy group. Furthermore, Shemirani et al.33 have shown that the thickness of the epicardial fat layer was significantly higher in the CAD group than in the normal group.
EFT thickness has been investigated in polycystic ovary syndrome, rheumatoid arthritis, lichen planus, hyperthyroidism, Type 2 diabetes, psoriasis, cardiovascular disease, inflammatory bowel disease, and chronic kidney disease; however, there is no study on its relationship with periodontal disease in the literature to the best of our knowledge.14,34–36
In this study, we found that EFT measured by echocardiography seems to be associated with severe periodontitis. The reason for high EFT measurements in the patient group can be attributed to the fact that periodontitis is a systemic inflammatory disease and the EFT values may increase in inflammatory diseases. Although it is known from previous studies that there is a higher relationship between EFT and atherosclerosis, such increased EFT values may indicate an increased risk of cardiovascular disease in patients with periodontitis.
LimitationsThe present case-control study has a small population size. Since the participants did not undergo coronary angiography, we were unable to directly demonstrate the relationship between epicardial adipose tissue thickness and coronary artery disease. Although cardiovascular magnetic resonance (MR) is the gold standard for imaging EFT thickness, we only performed the echocardiographic assessment.
ConclusionsIn our opinion, EFT thickness may be an indirect sign of cardiovascular diseases in patients with periodontitis. These results showed that periodontitis, a serious inflammatory disease, is also important in terms of cardiovascular health to maintain lifelong oral health by providing early diagnosis and treatment of a possible periodontal disease with regular dental check-ups. We need to support our findings with larger, prospective and randomized studies.
Informed consentInformed consent was obtained from all individual participants included in the study.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of interestThe authors have no conflicts of interest to declare.