Intermediate-high risk pulmonary embolism (IHR-PE) has a poor prognosis, but is under-represented in trials of direct oral anticoagulants (DOACs) in venous thromboembolic disease (VTE). We aimed to assess whether the administration of DOACs was equivalent to the conventional (CONV) treatment of low-molecular weight heparin bridged with warfarin for treating IHR-PE.
MethodsWe conducted a retrospective cohort study including 59 consecutive patients admitted with IHR-PE and followed for up to three months after discharge. Two groups were created based on the anticoagulant strategy: CONV (n=35) and DOAC (n=24). The efficacy endpoints were death, recurrent PE, estimated pulmonary artery systolic pressure (PASP), right ventricular systolic function (RVSF) at discharge, and length of stay; the safety endpoint was major bleeding.
ResultsThe two groups were similar regarding demographics, PE etiology and markers of clinical severity. There were four in-hospital deaths in the CONV group and none in the DOAC group. No recurrent PE or major bleeding event was recorded in either group. At discharge, neither PASP nor RVSF was different between the groups. Patients in the DOAC group were discharged 1.7 days earlier on average than patients in the CONV group (4.7±2.4 vs. 3.0±1.5 days, p=0.002).
ConclusionsThe adoption of a DOAC treatment strategy in this real-world cohort of IHR-PE patients was associated with similar efficacy and safety to the CONV approach. The fact that monitoring of anticoagulation effect was unnecessary probably led to the significant reduction in length of stay.
O tromboembolismo pulmonar de risco intermédio-elevado (TEP-IE) condiciona um prognóstico mais agravado, mas se encontra sub-representado nos ensaios dos anticoagulantes orais diretos (ACOd) na doença tromboembólica venosa (DTV). Avaliamos se a administração de ACOd foi equivalente à estratégia terapêutica convencional (CONV) (heparina de baixo peso molecular (HBPM) + varfarina) no tratamento do TEP-IE.
MétodosFez-se um estudo de coorte retrospetivo com 59 doentes consecutivos internados por TEP-IE, seguidos até três meses após a alta. Criaram-se dois grupos, baseados na estratégia terapêutica anticoagulante: CONV (n = 35) e DOAC (n = 24) (ACOd). Os desfechos de eficácia foram a morte, o TEP recorrente, a pressão sistólica na artéria pulmonar (PSAP), a função ventricular direita (FVD) e a duração do internamento; o desfecho de segurança foi a hemorragia major.
ResultadosOs grupos eram comparáveis relativamente aos aspetos demográficos, à etiologia do TEP e aos marcadores de gravidade clínica. Ocorreram quatro mortes intra-hospitalares no grupo CONV e nenhuma no grupo DOAC. Nenhum evento de TEP recorrente ou hemorragia major ocorreu em qualquer dos grupos. À data de alta, quer a PSAP quer a FVD não diferiram entre os dois grupos. A alta ocorreu 1,7 dia mais cedo no grupo DOAC do que no grupo CONV (4,7±2,4 versus 3,0±1,5 dias, p = 0,002).
ConclusõesA adoção de uma estratégia de tratamento com ACOd associou-se a um perfil de eficácia e segurança semelhante à abordagem convencional. A ausência da necessidade de monitoração do efeito anticoagulante provavelmente motivou a redução na duração de internamento.
Pulmonary embolism (PE) is a common disease, with an estimated annual incidence of 70 cases per 100 000.1,2 This condition can be life-threatening if not treated rapidly and appropriately and often leads to chronic disease and disability.3 Mortality from PE, 15% at three months, exceeds that for acute myocardial infarction.4 Risk stratification helps to optimize the selection of patients who will benefit from more aggressive therapy, such as thrombolysis or embolectomy, in addition to anticoagulation.4 High-risk patients are also most susceptible to the dreaded complication of chronic thromboembolic pulmonary hypertension.4 In order to predict early (in-hospital or 30-day) outcomes, both PE-related risk and the patient's clinical status and comorbidities should be taken into consideration, and can be measured using risk scores such as the pulmonary embolism severity index (PESI).5 Intermediate-high risk patients have positive cardiac laboratory biomarkers, signs of right ventricular (RV) dysfunction on an imaging test and PESI class III-V or simplified PESI (sPESI) ≥1.
The conventional PE treatment approach consists of a parenteral anticoagulant such as enoxaparin for at least five days, followed by a vitamin K antagonist (VKA) such as warfarin, and continued for at least three months.6 Although effective, this regimen is challenging, as enoxaparin requires daily subcutaneous injections and VKAs require close monitoring and dose adjustment.7 The recently developed direct oral anticoagulants (DOACs), which inhibit factor Xa or thrombin, can overcome these limitations.8 DOACs have been tested against conventional therapy in large phase III studies for the treatment of PE: EINSTEIN-PE for rivaroxaban, RE-COVER and RE-COVER II for dabigatran, AMPLIFY for apixaban and Hokusai-VTE for edoxaban.1,9–11 Rivaroxaban and apixaban allow for a single drug regimen, eliminating the need for initial parenteral anticoagulation, while dabigatran and edoxaban are initiated after a course of parenteral therapy.1 In all these studies, DOACs were shown to be at least as effective as VKAs for preventing recurrent VTE and VTE-related death, and demonstrated a similar or reduced incidence of major and/or major plus non-major clinically relevant bleeding (the principal safety outcome events), compared with conventional treatment.12 All DOACs have been approved for the treatment of PE and are recommended as alternatives to standard therapy in international guidelines.5,13 Although these recommendations are based on randomized clinical trials (RCTs),1,9–11 the real-world use of DOACs compared with warfarin is less clear. Phase III trials have selective inclusion criteria, and the reproducibility of their findings needs to be assessed in broader patient populations like those seen in routine clinical practice.14 Safety outcomes in clinical practice may diverge from trials for several reasons, including selective inclusion criteria and the limited duration of follow-up in the latter.15 Also, intermediate-high risk PE patients represent a particular subset of PE patients with a poorer prognosis,16 but are under-represented in RCTs and are not reported as an independent group in any of the PE RCTs. In this study we aimed to report the efficacy and safety of a DOAC-based regimen compared with a conventional treatment approach in patients admitted to a cardiac care unit with intermediate-high risk PE.
MethodsWe conducted a single-center, retrospective cohort study of patients with confirmed PE treated with the standard anticoagulation regimen (CONV) or with DOACs. The study was approved by the local ethics committee and was in accordance with the 2008 Declaration of Helsinki.
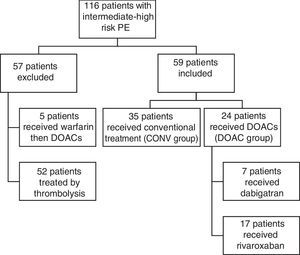
Study populationBetween January 2010 and August 2015, 116 consecutive patients admitted with intermediate-high risk PE were included. Patients with intermediate-high risk PE were defined as those with positive myocardial lesion markers (troponin I), right ventricular dysfunction (assessed by echocardiography), and PESI class III-V or sPESI ≥1. After the introduction of DOACs in our hospital's pharmacy (in August 2013), all PE patients with indication and without contraindication were started on DOACs for treatment of acute PE. We retrospectively selected a historical cohort of PE patients with similar clinical characteristics treated with a CONV approach between February 2010 and September 2014. A total of 57 patients initially treated by thrombolysis were excluded from the analysis (Figure 1).
The choice of DOAC was left to the discretion of the attending physician. The treatment regimens were as recommended by the manufacturer.1 Discharge reports were used to obtain information on demographics, personal history and laboratory workup. All patients were followed for up to three months after discharge.
ExposurePatients with intermediate-high risk PE were managed either with a conventional approach (enoxaparin followed by VKA) (CONV group, n=35) or with DOACs (DOAC group, n=24) (Figure 1).
EndpointsThe primary efficacy endpoints were (i) death, (ii) recurrent PE, (iii) echocardiographically-estimated pulmonary artery systolic pressure (PASP) at discharge, (iv) right ventricular function at discharge and (v) length of stay. Death was sub-classified as being PE-related, bleeding-related, or from other causes. Recurrent PE was defined as the new onset of symptoms confirmed by diagnostic testing. PASP and right ventricular function were obtained from the discharge echocardiogram report. The primary safety endpoint was major bleeding. Major bleeding was defined as overt bleeding associated with a fall in hemoglobin of 2g/l or more; a transfusion of two or more units of packed red blood cells; critical site bleeding (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal); or fatal bleeding (defined using the International Society on Thrombosis and Haemostasis criteria).17
Statistical analysisData are shown as means ± standard deviation (SD) for continuous variables and absolute and relative frequencies (%) for categorical variables. The paired-sample t test and the sign test were used to compare treatment strategies. All analyses were performed with STATA 12.0 (StataCorp LP, TX, USA). A two-sided p<0.05 was considered statistically significant.
ResultsThe clinical characteristics of the study population are shown in Table 1. Of the 59 patients included, 35 (59%) were managed with a CONV approach and 24 (40%) with a DOAC approach. The mean patient age was 62±19 years (median 58, range 29-87) and 61±17 years (median 57, range 24-78) for the CONV and DOAC groups, respectively (p=0.770). No differences were found between the CONV and DOAC groups in gender or laboratory tests. Creatinine clearance was similar (p=0.881). Regarding cardiac biomarkers, no significant differences were found between the two groups, although B-type natriuretic peptide (BNP) levels were numerically higher in the CONV group (Table 1).
Demographic and clinical characteristics of the study population.
| Variable | CONV (n=35) | DOAC (n=24) | p |
|---|---|---|---|
| Age, years | 62±19 | 61±17 | 0.770 |
| Male, n (%) | 13 (37) | 9 (37) | -- |
| CrCl, n (%) | |||
| <30 ml/min | 1 (2.9) | 0 (0) | |
| 30 to <50 ml/min | 5 (14.3) | 2 (8.3) | 0.881 |
| 50 to <80 ml/min | 10 (28.6) | 10 (41.7) | |
| ≥80 ml/min | 19 (54.2) | 12 (50) | |
| Diagnostic method and classification | |||
| CTPA, n (%) | 35 (100) | 24 (100) | -- |
| Laboratory tests | |||
| D-dimers, μg/ml (n) | 7.93 (30) | 8.44 (23) | 0.897 |
| BNP, pg/ml (n) | 420.17 (23) | 163.46 (11) | 0.095 |
| TnI, ng/ml (n) | 0.75 (35) | 0.57 (24) | 0.717 |
| Myoglobin, ng/ml (n) | 116.09 (35) | 53.79 (24) | 0.138 |
| CK, U/L (n) | 128.73 (33) | 90.88 (24) | 0.337 |
| CK-MB, U/L (n) | 2.45 (35) | 2.73 (24) | 0.778 |
| PE risk factors, n (%) | |||
| Unprovoked | 17 (49) | 21 (87) | |
| Provoked | 11 (31) | 3 (13) | 0.030 |
| Not reported | 7 (20) | 0 | |
| Previous VTE, n | 2 | 0 | -- |
| Active cancer, n | 1 | 1 | -- |
| Known thrombophilia, n | 0 | 0 | -- |
| Pregnancy, n | 2 | 0 | -- |
BNP: B-type natriuretic peptide; CK: creatine kinase; CONV: conventional anticoagulation group; CrCl: creatinine clearance; CTPA: computed tomography pulmonary angiography; DOAC: direct oral anticoagulant group; PE: pulmonary embolism; TnI: troponin I; VTE: venous thromboembolism.
In the DOAC group, three cases of PE were provoked (cancer, surgery and prolonged immobilization), while 11 in the CONV group were provoked (childbirth, chronic pulmonary embolism, cancer, surgery and immobilization). None of the patients in the DOAC group had a prior history of PE or known thrombophilia, while in the CONV group, two patients had a prior PE (these patients had a history of cancer); none had known thrombophilia.
OutcomesThere were four in-hospital death in the CONV group, whereas no deaths occurred in the DOAC group. No recurrent PE was recorded in any of the groups during admission or during the three-month follow-up. Regarding the safety outcome, no major bleeding events were observed in either group. There was a statistically significant difference (p=0.002) between the CONV (4.7±2.4) and the DOAC (3.0±1.5) groups in length of stay. No differences were found regarding echocardiographic parameters at discharge, including PASP (p=0.471) and right ventricular systolic function (p=0.684) (Table 2).
Clinical outcomes during the treatment period.
| Variables | CONV (n=35) | DOAC (n=24) | p |
|---|---|---|---|
| Efficacy endpoints | |||
| Death, n | 4 | 0 | |
| Recurrent PE, n | 0 | 0 | |
| PASP (mmHg), mean ± SD | 44.9±8.4 | 41.2±9.3 | 0.471 |
| RV function, n (%) | |||
| Normal | 10/24 (41.8) | 7/23 (30.4) | |
| Mildly depressed | 7/24 (29.1) | 9/23 (39.2) | 0.684 |
| Moderately/severely depressed | 7/24 (29.1) | 7/23 (30.4) | |
| LOS (days), mean ± SD | 4.7 ± 2.4 | 3.0 ± 1.5 | 0.002 |
| Safety endpoint | |||
| Major bleeding, n | 0 | 0 | |
CONV: conventional anticoagulation group; DOAC: direct oral anticoagulant group; LOS: length of stay; PASP: pulmonary artery systolic pressure; PE: pulmonary embolism; RV: right ventricular.
The demand for real-world data in addition to clinical trial data is increasing.17 Non-interventional studies are crucial for obtaining a different perspective on the safety and effectiveness of drugs when used in routine clinical practice. Studies performed with real-world populations complement the outcomes of pivotal trials through the use of unselected real-world populations and conditions. Here, we report a real-world observational study analyzing the use of DOACs in the treatment of intermediate-high risk PE. We show that the efficacy and safety of such a strategy is comparable to the conventional, VKA-based approach and that length of stay may be reduced with a DOAC-based regimen, a critical finding in the intensive care unit setting.
DOACs have been tested against conventional therapy for the treatment of PE in large phase III trials: EINSTEIN-PE for rivaroxaban, RE-COVER and RE-COVER II for dabigatran, AMPLIFY for apixaban and Hokusai-VTE for edoxaban. In all these these trials, DOACs were shown to be at least as effective as VKAs for preventing recurrent VTE and VTE-related death, and demonstrated a similar or reduced incidence of major and/or major plus non-major clinically relevant bleeding (principal safety outcome events), compared with conventional treatment. A meta-analysis of the phase III VTE trials concluded that DOACs and VKAs have similar efficacy in the treatment of acute symptomatic VTE, a finding that is consistent in key clinical subgroups, and concluded that treatment with a DOAC significantly reduces the risks of major bleeding.18 However, intermediate-high risk PE defines a population at higher risk of both thrombotic and bleeding complications that is not well represented in most trials of DOACs in VTE, as most do not include information on cardiac necrosis markers or right ventricular function. Therefore, real-world treatment data on this specific stratum of PE patients are paramount.
Our study shows similar rates of death and recurrent PE during hospital stay and follow-up in both groups. Additionally, no major bleeding events were observed within three months of beginning treatment. These results highlight the safety of anticoagulation in this population and are consistent with those of RCTs on DOACs, in which the incidence of major bleeding was around 1% for all studies. In other studies in the real-world setting, the risk of bleeding related to DOACs was similar to that for warfarin.19 Reassuringly, the findings of our study are in line with those in the literature for patients treated in routine clinical practice.20
Another important point relates to the efficacy of DOACs for thrombus disintegration in intermediate-high risk PE patients, who may have a higher thrombotic burden in the lung vasculature. It is suggested that DOACs may be even more effective than warfarin in patients with markers of RV dysfunction, as shown in the Hokusai-VTE trial. However, in this trial RV dysfunction was analyzed only by elevation of BNP, a criterion that does not reflect usual clinical practice, which is to integrate laboratory, imaging and clinical risk score data.11
In line with the evidence from RCTs,11 we found no differences between the groups in two surrogate markers of right ventricular afterload, echocardiographically-estimated PASP and right ventricular function. It is therefore plausible that thrombus resolution rate and extent would be similar in both treatment regimens.
We found a significant difference between the two groups in length of stay, which was more than one day shorter in the DOAC group. This finding is probably due to the fact that the maximum anticoagulant effect of warfarin is only achieved 3-5 days after the first dose, and dosage needs to be adjusted according to the international normalized ratio (INR) before the patient is discharged. With the DOAC treatment strategy, after the patient is stabilized in terms of heart failure, oxygen saturation and pain (which frequently occurs before the target INR is achieved), there is no need to prolong hospitalization.
Among study limitations, we highlight the study's retrospective design and the use of a historical cohort of patients treated with VKAs, the small number of patients enrolled, related to the early experience using DOACS in treating PE, and the absence of thrombotic or bleeding events, a fact that reflects the very good prognosis of correctly treated PE, even those of intermediate-high risk.
ConclusionsThe adoption of a DOAC treatment strategy in intermediate-high risk PE patients in this typical real-world cohort was associated with comparable efficacy and safety to the conventional treatment regimen of parenteral anticoagulant plus warfarin. Importantly, length of stay was significantly reduced. No differences were found regarding right ventricular function markers at discharge. Further studies in larger cohorts will help to clarify the use of DOACs in this challenging PE population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestR.B. has received speaker fees from BMS/Pfizer, Bayer and Daiichi-Sankyo, investigator fees from Bayer and personal fees (advisory board) from BMS/Pfizer and Bayer.
S.M. has received speaker fees from BMS/Pfizer and Bayer, investigator fees from Bayer and personal fees (advisory board) from Bayer.
P.M. has received speaker fees from BMS/Pfizer, Bayer, Daiichi-Sankyo and Boheringer-Ingelheim, investigator fees from Bayer, Boheringer-Ingelheim and Daiichi-Sankyo and personal fees (advisory board) from BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo.