Fabry disease is caused by intracellular accumulation of glycosphingolipids in various tissues, secondary to mutations in the GLA gene (Xq22).
Classically described as affecting hemizygous males with no residual alpha-galactosidase A activity, it is now known to affect both sexes, with later and less severe manifestations in females. The manifestations of this disease are systemic: neurological, cutaneous (angiokeratomas), renal, cardiovascular (left ventricular hypertrophy, valve thickening or rhythm disturbances), cochlear-vestibular, and cerebrovascular. In the absence of treatment there is progressive damage to vital organs with renal failure, stroke, heart failure or rhythm perturbations, leading to severe impairment of quality of life as well as reduced life expectancy.
We describe the case of a female patient with a history of cryptogenic ischemic stroke at the age of 38 years, and chronic renal failure with proteinuria, who presented to the emergency room with atrial fibrillation. The echocardiogram revealed concentric left ventricular hypertrophy, diastolic dysfunction and decreased longitudinal strain in the basal septum.
In the context of a screening protocol, she was diagnosed with Fabry disease and a previously undescribed mutation was identified.
A doença de Fabry é causada por acumulação intracelular de glicoesfingolipidos em vários tecidos, secundária a mutações no gene GLA (Xq22).
Classicamente descrita como afectando homens hemizigotos sem actividade residual de alfa-galactosidase-A, sabe-se hoje afectar ambos os sexos com manifestações mais tardias e frustres no feminino. Associa-se a sintomas neurológicos, cutâneos (angioqueratomas), renais, cardiovasculares (hipertrofia ventricular esquerda, alterações do ritmo e espessamento valvular), cocleo-vestibulares, e cerebrovasculares.
Na ausência de tratamento ocorre dano progressivo de órgãos vitais: doença renal terminal e complicações cardiovasculares e cerebrovasculares potencialmente fatais, implicando redução da qualidade e esperança de vida.
Descreve-se o caso de doente do sexo feminino, com antecedentes de acidente vascular cerebral (AVC) isquémico criptogênico com 38 anos e insuficiência renal crónica (IRC) com proteinúria, que se apresenta no serviço de urgência com fibrilhação auricular. O estudo ecocardiográfico revelou hipertrofia concêntrica do ventrículo esquerdo com disfunção diastólica e diminuição do strain longitudinal basal. Englobada num protocolo, de rastreio, foi-lhe diagnosticada doença de Fabry e identificada uma mutação não descrita previamente.
Fabry disease (FD) is the second most prevalent lysosomal storage disorder after Gaucher disease. It is caused by the progressive accumulation of globotriaosylceramide (Gb3) due to deficiency of the lysosomal hydrolase alpha-galactosidase A (α-Gal A), leading to progressive dysfunction of various organs. α-Gal A catalyzes the hydrolytic cleavage of the terminal galactose from Gb31.
Over 350 mutations in the GLA gene, on the long arm of chromosome X (Xq22), have been described. Many are private (restricted to one or a few families), and around 10% are new mutations2.
Inheritance of FD is X-linked: hemizygous males develop the classic disease, while heterozygous females have milder manifestations and later onset, presumably because the X chromosome with the normal allele is randomly inactivated.
PrevalenceThe estimated prevalence is 1:17 000 to 1:117 000 in Caucasian men, but the wide range of clinical manifestations (often nonspecific), and the fact that most clinicians have limited experience of FD, mean that these Figures could be underestimates. Newborn screening found a prevalence of 1: 40003.
Screening of specific populations reveals higher prevalences: 0.16-1.2% in patients with chronic renal failure (CRF) undergoing hemodialysis4; 4.9% in men and 2.4% in women aged under 50 with cryptogenic stroke5; and up to 7% in men and 12% in women with unexplained left ventricular hypertrophy (LVH)6.
Clinical presentationAlthough most symptoms begin in childhood, clinical diagnosis can take decades or indeed never be made. In males the first manifestations of the disease usually occur between the ages of four and ten, with acroparesthesia and pain, fever, hypohidrosis, heat intolerance, gastrointestinal symptoms (diarrhea, nausea and vomiting), cutaneous angiokeratomas and corneal alterations. Proteinuria and neurological manifestations appear from the second decade of life, while cardiac involvement is only seen from the third and fourth decades and is responsible for much of the associated morbidity and mortality. In females the manifestations occur later and can be milder6,7.
Cardiac involvementGb3 accumulates in cardiomyocytes, conduction system cells, valvular fibroblasts, endothelial cells and vascular smooth muscle cells. However, chronic accumulation of Gb3 accounts for only 1-2% of total cardiac mass; it is postulated that it causes cell dysfunction by interfering with signaling pathways, leading to hypertrophy, apoptosis, necrosis and fibrosis. Ischemia resulting from deposition in the vascular endothelium and mitochondrial dysfunction may also play a part.
In the Fabry Outcome Survey (FOS), cardiac symptoms (including angina, dyspnea, palpitations and syncope) were reported in 69% of affected men and 65% of affected women8. LVH is the most common cardiac manifestation of FD, correlating with age in both sexes, but occurring earlier in men. There is an inverse relation between renal function and degree of ventricular hypertrophy8. Although the pattern of hypertrophy is typically concentric, in 5% of cases it is asymmetric and can mimic hypertrophic cardiomyopathy (HCM)9. The presence of LVH is associated with a higher prevalence of cardiac symptoms, arrhythmias and valve disease.
The ECG shows voltage criteria for LVH and/or ventricular repolarization abnormalities, which may appear earlier6.
Two-dimensional echocardiography is the exam of choice to assess left ventricular wall thickness and morphology. Endocardium with a binary appearance has been proposed as a specific and sensitive marker for FD in patients with LVH, but there is disagreement surrounding this suggestion and considerable inter-observer variability10. Right ventricular hypertrophy is found in a third of cases in association with LVH; it affects both sexes and increases in frequency with age. Left ventricular diastolic function is frequently affected, with 25% of patients presenting relaxation abnormalities. A pseudonormal pattern is less common and a restrictive pattern is rare.
Carriers of a mutation have reduced Sa, Ea and Aa velocities at the septal and lateral mitral annulus before developing hypertrophy, which continue to deteriorate as the latter worsens. The role of tissue strain rate and speckle tracking is still under study.
Detection of areas of fibrosis by cardiac magnetic resonance imaging with gadolinium delayed enhancement identifies patients with worse response to enzyme replacement therapy (ERT) and worse prognosis.
Although there is valve involvement in 14.6% of cases, with fibrosis, calcification and dysfunction, valve replacement is rarely required.
The epicardial coronary arteries are obstructed in only a minority of patients, but anginal symptoms are common; the underlying mechanisms are thought to be microvascular and endothelial dysfunction, reduced coronary flow reserve and increased oxygen demand by the hypertrophied myocardium6.
TreatmentAs multiple organ systems are involved in FD, a multidisciplinary approach is essential.
From a cardiac standpoint, treatment of angina and heart failure is based on the same principles as for other patients, with particular attention to the bradycardic effect of beta-blockers and non-dihydropyridine calcium channel blockers. In some patients coronary angiography may be indicated to exclude significant epicardial coronary disease. In cases of end-stage heart failure, heart transplantation is a viable option, since ERT prevents accumulation of Gb3 in the graft9. Oral anticoagulation should always be considered in patients with atrial fibrillation.
Sotalol is the antiarrhythmic of choice, as amiodarone interferes with lysosomal metabolism. Pacemaker implantation should follow the general guidelines and a cardioverter-defibrillator should be implanted in those at risk of sudden death. Although there are no clear recommendations, some authors suggest that the criteria used for HCM should be extended to FD.
Two ERT formulations have been in use since 2001: agalsidase-α, produced from a modified human cell line, and agalsidase-β, produced from Chinese hamster ovary cells. Both are administered fortnightly by intravenous infusion and are well tolerated, with only mild infusion-related reactions. ERT reduces Gb3 levels in cardiac tissue, particularly in vascular endothelial cells, and can lead to regression of LVH and improved regional myocardial strain11.
Clinical research has revealed molecules that increase residual enzymatic activity (chaperones) or reduce the formation of Gb3 (substrate inhibitors) and thus have the potential to prevent or reverse clinically significant deposition of Gb3.
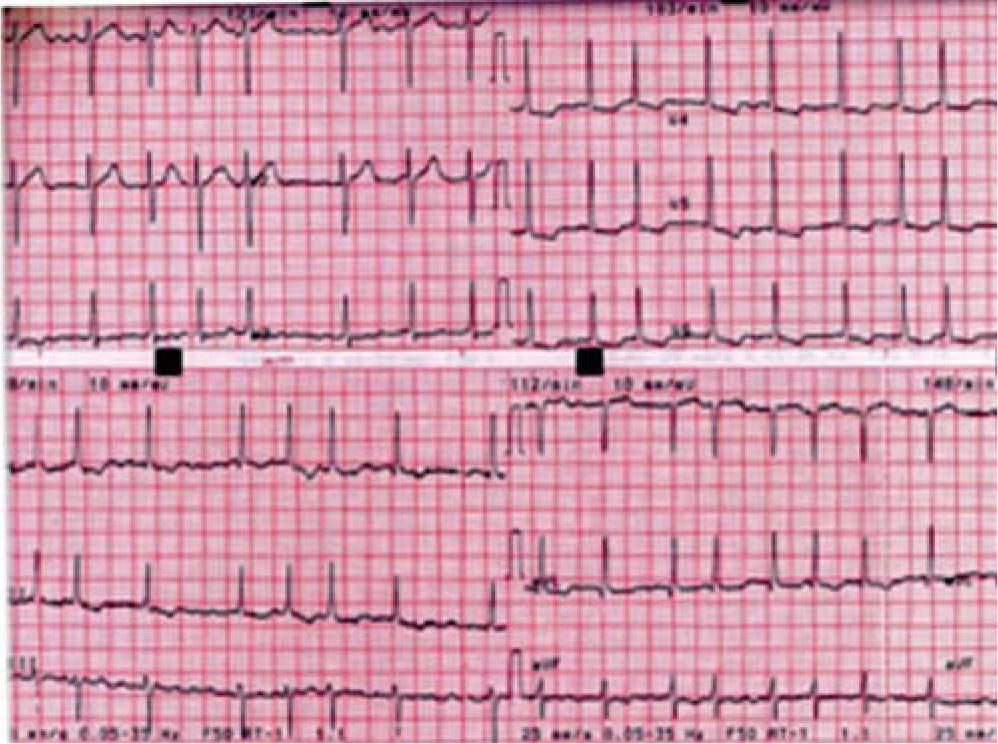
Case reportA 48-year-old woman went to the ER with palpitations. She was in atrial fibrillation (AF) with rapid ventricular response (110–120bpm) (Figure 1).
Pharmacological conversion was attempted with a 48-hour amiodarone perfusion and digoxin to control heart rate, under anticoagulation therapy, but she continued in AF with a controlled ventricular response of around 80bpm. She was discharged medicated with beta-blockers and was scheduled for later electrical cardioversion.
The admission ECG (Figure 1) showed ventricular response of 110bpm; flattened T waves in I, aVL, II and III; ST-segment depression; and asymmetric T wave inversion in V4-V6, suggestive of left ventricular overload.
Her personal history included worsening hearing loss over the previous eight years, requiring a hearing aid; hypertension diagnosed at the age of 38; cryptogenic ischemic stroke at the age of 38; treatment for depression nine years previously; and degenerative osteoarthritis. She reported no cutaneous lesions, intolerance to heat or cold, abdominal pain or gastrointestinal abnormalities.
She had been followed in the nephrology clinic for two years with stage 2 CRF, hypertension and subnephrotic proteinuria, controlled by angiotensin-converting enzyme inhibitors. In the course of assessment of CRF she underwent renal biopsy, which suggested hypertensive nephroangiosclerosis.
Family history included the father's death at the age of 76 from unknown cause; the mother's nocturnal sudden death at the age of 31; and a 46-year-old brother with kidney transplantation 10 years previously and cardiac problems since childhood.
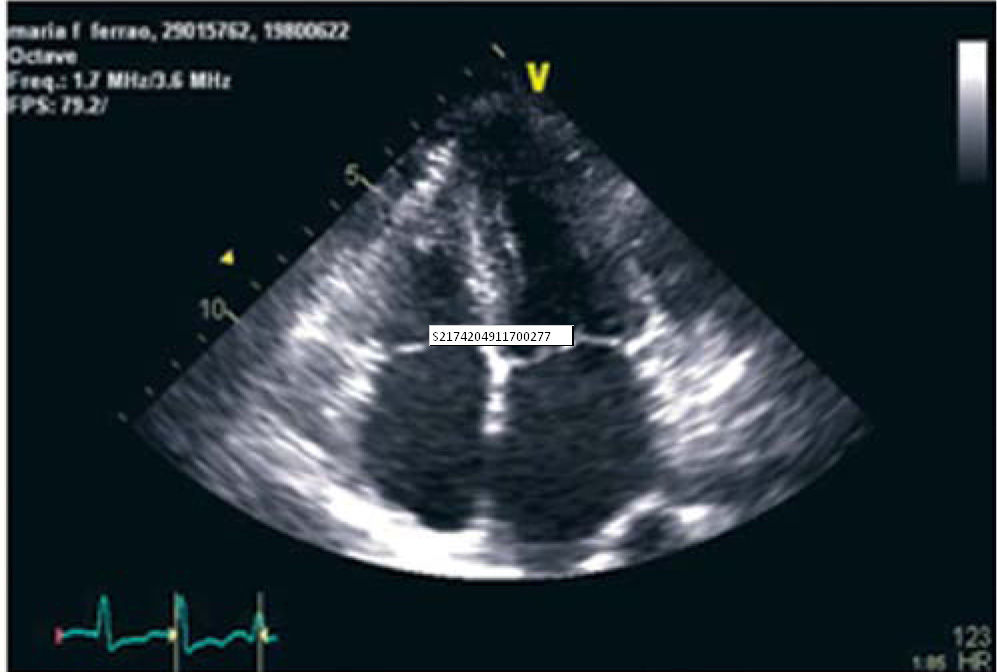
The echocardiogram revealed slight thickening of the mitral, aortic and tricuspid valves; a mild central regurgitation jet was visualized at the aortic valve, as well as two mild mitral regurgitation jets. There was moderate tricuspid regurgitation, with pulmonary artery systolic pressure estimated at 34mmHg. Mild to moderate dilatation of the left atrium was seen, while the right ventricle presented normal dimensions, with slightly hypertrophied walls. The left ventricle had normal dimensions and preserved systolic function (ejection fraction of 60% by the Simpson biplane method). The echogenicity of the endocardium was increased and the myocardium was thickened (intraventricular septum 13mm, posterior wall 12mm) (Figure 2).
Assessment of diastolic function revealed altered relaxation and an E/Em ratio of 14.4. Diastolic velocities of the annular myocardium were reduced at the interventricular septum (Em 7mm/s) and were at the lower limit of normality at the lateral wall (Em 10mm/s).
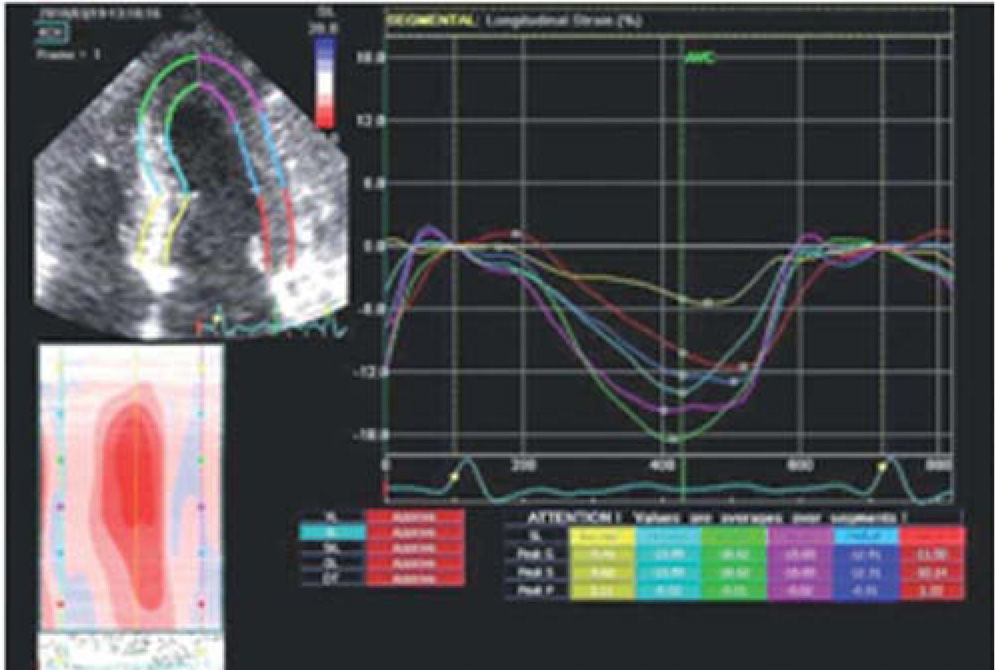
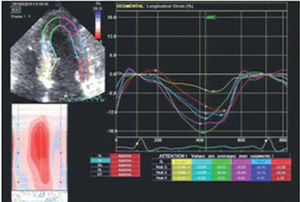
Longitudinal strain increased from the base to the apex in the septal and lateral walls and was severely reduced (−5.41%) at the basal septum (Figure 3).
Given the clinical suspicion of FD, alpha-galactosidase A was measured in dried blood spot samples. The value of 0.33 heightened the suspicion, and genotyping revealed a Glu358 deletion in the GLA gene, compatible with FD.
The patient is currently medicated with ramipril plus diuretic, carvedilol, warfarin, simvastatin and paroxetine.
She has been referred for ERT and is currently being assessed by the National Coordinating Center for Diagnosis and Treatment of Lysosome Disorders.
DiscussionFabry disease is caused by deficient production or functioning of the alpha-galactosidase A enzyme, which leads to the accumulation of Gb3 in various tissues and organs and their progressive dysfunction. Although rare, it should be considered by the clinician faced with renal, cardiac and cerebrovascular dysfunction in young adults.
Our patient presented several typical cardiac manifestations of the disease: valve thickening with mild regurgitation, myocardial hypertrophy of both ventricles, and rhythm disturbances (atrial fibrillation with relatively controlled ventricular response of 110bpm at admission). Analysis of longitudinal myocardial strain revealed a pattern similar to that described for HCM.
The patient also presented renal involvement, hearing loss and typical central nervous system manifestations. The fact that she is a woman and had almost normal a-Gal A levels does not invalidate the diagnosis: even in women with normal enzyme levels, mutations in the GLA gene should be investigated.
The fact that a previously unknown mutation was found is not surprising given that in most families the disease results from a private mutation.
This case highlights a common problem with Fabry disease: the long delay between symptom onset and diagnosis (ten years in this case). Only by considering FD as a differential diagnosis in young patients with stroke, proteinuria or unexplained left ventricular hypertrophy can a timely diagnosis be made and appropriate treatment begun.
Conflicts of interestThe authors have no conflicts of interest to declare.